
What are the materials required for the experiment to show chemical decomposition of water? Write the procedure of the experiment. Name the products we get in this reaction.
Answer
573.6k+ views
Hint: To decompose very stable compounds like water electrolysis is a suitable method. When current is passed through the solution of a compound, it ionizes the compound and now these newly formed ions will move towards anode and cathode according to their charges. Positively charged ions will move towards cathode and negatively charged ions will move towards anode. At cathode positively charged ions will gain electrons and get discharged and at anode negatively charged ions lose the electron and get discharged.
Complete step by step solution:
Chemical decomposition of water will be done by electrolysis.
To perform electrolysis of water we need pencil, battery, electrolytic cell, connecting wire, distilled water, cardboard and very small quantities of acid.
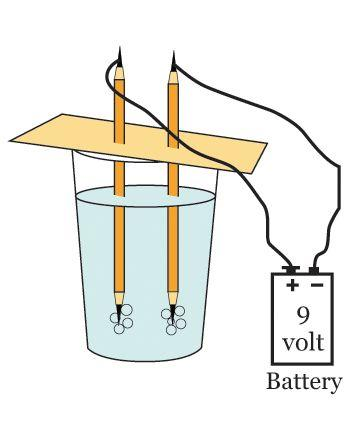
- We will have the set up as shown in the diagram. We will fill the beaker with distilled water and with help of cardboard arrange the pencils as an electrode. Pencils will work as an electrode because their lead is made of graphite which is a good conductor of electricity. We will connect these graphite electrodes with the battery by using a connecting wire. Very small quantity of acid is used as a precursor for this electrolysis process.
- When we will switch on the battery and let the current pass, the following reaction in the acidic medium is going to take place.
At cathode: \[2{{H}^{+}}+2{{e}^{-}}\to {{H}_{2}}\]
At anode: $2{{H}_{2}}O\to {{O}_{2}}+4{{H}^{+}}+4{{e}^{-}}$
Net reaction: $2{{H}_{2}}O\to {{O}_{2}}+2{{H}_{2}}$
At cathode reduction of ${{H}^{+}}$ ion is taking place and ${{H}_{2}}$ gas is forming at cathode
At anode oxidation of $O{{H}^{-}}$ ion is taking place and ${{O}_{2}}$ gas is forming at anode.
So products in this reaction are ${{H}_{2}}$ and ${{O}_{2}}$
Additional Information:
Electrolysis of water can be done in the solution containing trace quantities of salts but for that salt selection is very important. Otherwise salt cation and anion will become competitors and themselves get discharged. Salts having lesser standard electrode potentials than hydrogen and hydroxide ions are suitable for the electrolysis of water.
Note: The efficiency of electrolysis or the electron transfer depends on many factors such as;
i) The number cations and anions in the solution
ii) Mobility of the ions
iii) Activation energy needed for the electron transfer from the electrode to the electrolyte ions.
Complete step by step solution:
Chemical decomposition of water will be done by electrolysis.
To perform electrolysis of water we need pencil, battery, electrolytic cell, connecting wire, distilled water, cardboard and very small quantities of acid.

- We will have the set up as shown in the diagram. We will fill the beaker with distilled water and with help of cardboard arrange the pencils as an electrode. Pencils will work as an electrode because their lead is made of graphite which is a good conductor of electricity. We will connect these graphite electrodes with the battery by using a connecting wire. Very small quantity of acid is used as a precursor for this electrolysis process.
- When we will switch on the battery and let the current pass, the following reaction in the acidic medium is going to take place.
At cathode: \[2{{H}^{+}}+2{{e}^{-}}\to {{H}_{2}}\]
At anode: $2{{H}_{2}}O\to {{O}_{2}}+4{{H}^{+}}+4{{e}^{-}}$
Net reaction: $2{{H}_{2}}O\to {{O}_{2}}+2{{H}_{2}}$
At cathode reduction of ${{H}^{+}}$ ion is taking place and ${{H}_{2}}$ gas is forming at cathode
At anode oxidation of $O{{H}^{-}}$ ion is taking place and ${{O}_{2}}$ gas is forming at anode.
So products in this reaction are ${{H}_{2}}$ and ${{O}_{2}}$
Additional Information:
Electrolysis of water can be done in the solution containing trace quantities of salts but for that salt selection is very important. Otherwise salt cation and anion will become competitors and themselves get discharged. Salts having lesser standard electrode potentials than hydrogen and hydroxide ions are suitable for the electrolysis of water.
Note: The efficiency of electrolysis or the electron transfer depends on many factors such as;
i) The number cations and anions in the solution
ii) Mobility of the ions
iii) Activation energy needed for the electron transfer from the electrode to the electrolyte ions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




