
How are the following conversions carried out? 2-Methyl-1-propene to 2-chloro-2-methylpropane
Answer
565.2k+ views
Hint: In chemistry, carbon is a very versatile element that has a tetravalent structure which means it can attach to mostly four elements at a time, but it also shows a wide variety of bond structure ranging from the single bond, double bond, and triple bond. All these bonds take different types of strengths and exist with different electrons which are in participation.
Complete step by step answer:
Carbon is a very versatile element and showcases a wide range of bond types ranging from single to triple. All these bonds exist but the properties of these bonds are very different. Carbon is said to be most stable in its quaternary state which means the carbon is the most stable when it is surrounded by four other elements. This is possible only when all the elements are connected to the central atom through a single bond.
The conversion given in the question is an example of the process of chlorination. It is the process in which the chlorine atom is attached to the given compound. The initial compound usually has multiple bonds that break to make way for the chlorine atom to get attached to the compound.
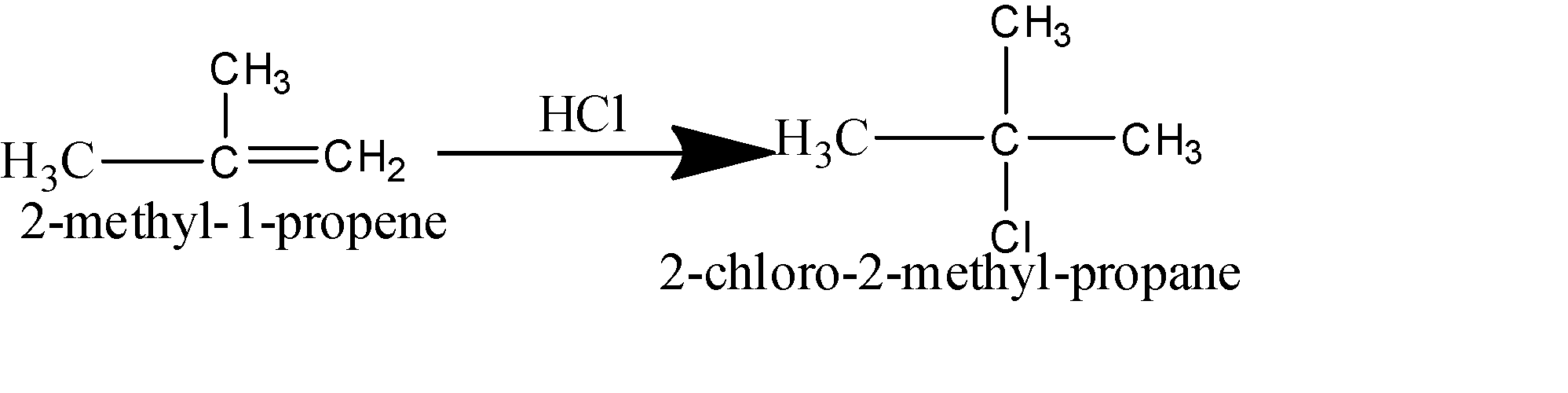
Note: Carbon is a tetravalent atom which means it has four valence electrons and thus can form four covalent bonds with atoms.
Based on this property the carbon atom can be categorized as primary, secondary, or tertiary
The carbon which is attached to only one other carbon atom is called primary carbon, the carbon atom with the attachment of two other carbon atoms is secondary carbon whereas the carbon atom with the attachment of three carbon atoms is termed as a tertiary carbon atom.
Complete step by step answer:
Carbon is a very versatile element and showcases a wide range of bond types ranging from single to triple. All these bonds exist but the properties of these bonds are very different. Carbon is said to be most stable in its quaternary state which means the carbon is the most stable when it is surrounded by four other elements. This is possible only when all the elements are connected to the central atom through a single bond.
The conversion given in the question is an example of the process of chlorination. It is the process in which the chlorine atom is attached to the given compound. The initial compound usually has multiple bonds that break to make way for the chlorine atom to get attached to the compound.

Note: Carbon is a tetravalent atom which means it has four valence electrons and thus can form four covalent bonds with atoms.
Based on this property the carbon atom can be categorized as primary, secondary, or tertiary
The carbon which is attached to only one other carbon atom is called primary carbon, the carbon atom with the attachment of two other carbon atoms is secondary carbon whereas the carbon atom with the attachment of three carbon atoms is termed as a tertiary carbon atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




