
What are the 9 isomers of Heptane?
Answer
517.8k+ views
Hint: Isomers are those compounds which have the same molecular formula but have different spatial arrangement. Heptane is an alkane having seven carbon atoms. Hence, the chemical formula for heptane is \[C_7H_{16}\].
Complete answer:
The chemical formula for heptane is \[C7H16\]. The structure of first isomer named as n-heptane is given as follows:
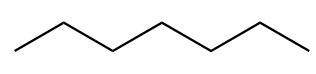
The structure of second isomer named as 2-Methylhexane is given as follows:
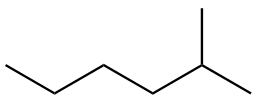
The structure of third isomer named as 3-Methylhexane is given as follows:
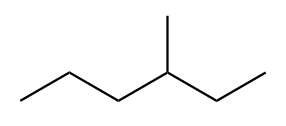
The structure of fourth isomer named as 2,2-Dimethylpentane is given as follows:
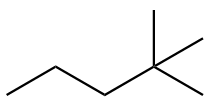
The structure of fifth isomer named as 2,3-Dimethylpentane is given as follows:
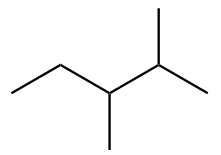
The structure of sixth isomer named as 2,4-Dimethylpentane is given as follows:
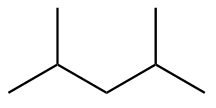
The structure of seventh isomer named as 3,3-Dimethylpentane is given as follows:
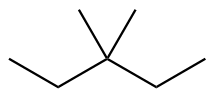
The structure of eighth isomer named as 3-Ethylpentane is given as follows:
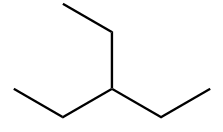
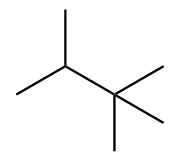
The structure of ninth isomer named as 2,2,3-Trimethylbutane is given as follows:
From the above structures, we can see that all structures differ in structural arrangement in space and have the same molecular formula.
Therefore, the 9 isomers of heptane are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Note:
It is important to note that isomers have the same chemical formula but have different structures. The 9 isomers of heptane differ in the number of carbon atoms in the parent chain. The IUPAC name of these isomers are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Complete answer:
The chemical formula for heptane is \[C7H16\]. The structure of first isomer named as n-heptane is given as follows:

The structure of second isomer named as 2-Methylhexane is given as follows:

The structure of third isomer named as 3-Methylhexane is given as follows:

The structure of fourth isomer named as 2,2-Dimethylpentane is given as follows:

The structure of fifth isomer named as 2,3-Dimethylpentane is given as follows:

The structure of sixth isomer named as 2,4-Dimethylpentane is given as follows:

The structure of seventh isomer named as 3,3-Dimethylpentane is given as follows:

The structure of eighth isomer named as 3-Ethylpentane is given as follows:

The structure of ninth isomer named as 2,2,3-Trimethylbutane is given as follows:

From the above structures, we can see that all structures differ in structural arrangement in space and have the same molecular formula.
Therefore, the 9 isomers of heptane are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Note:
It is important to note that isomers have the same chemical formula but have different structures. The 9 isomers of heptane differ in the number of carbon atoms in the parent chain. The IUPAC name of these isomers are n-heptane, 2-Methylhexane, 3-Methylhexane, 2,2-Dimethylpentane, 2,3-Dimethylpentane, 2,4-Dimethylpentane, 3,3-Dimethylpentane, 3-Ethylpentane and 2,2,3-Trimethylbutane.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




