
How are lanthanides extracted from monazite sand?
Answer
590.1k+ views
Hint: All the lanthanides look like silvery-white soft metals and in presence of air tarnish rapidly. The separation of lanthanides from other elements forms lanthanide compounds which are chemically combined with specific substances. There are more methods available used for separation of lanthanides like monazite sand, bastnasite, xenotime, and other minerals.
Complete step by step solution:
Monazite sand: this is a phosphate of thorium and various earth minerals like lanthanum and available in sand or gravel deposits which is a yellow to red or brown mineral.
Monazite sand contains about 13.44% lanthanum oxide which is to be recovered. The basic principle of extracting lanthanum oxide from the monazite of the Momeik Myitsone Area.
The extraction of lanthanum from monazite follows three mains steps,
(1) By using caustic soda, the extraction of lanthanum hydroxide from monazite
(2) Digestion with nitric acid, and
(3) Precipitation with ammonium hydroxide and calcinations of lanthanum oxalate to lanthanum oxide
Experimental procedure:
Monazite sand is a raw material for this experimental procedure. Monazite to obtain 325 meshes which are finely ground and beach colour. The flow diagram of the extraction of lanthanum oxide is shown below.
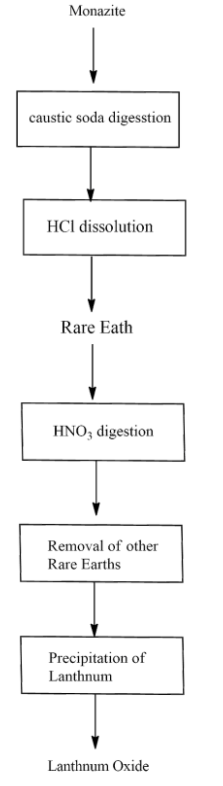
Note: Individual lanthanides are applied to separate by physical method. The anhydrous fluorides and chlorides are heated under an atmosphere of argon in presence of calcium at 1270K to get individual lanthanum metal. The pure metals are obtained by heating the trifluorides of lanthanides in the presence of calcium and lithium.
Complete step by step solution:
Monazite sand: this is a phosphate of thorium and various earth minerals like lanthanum and available in sand or gravel deposits which is a yellow to red or brown mineral.
Monazite sand contains about 13.44% lanthanum oxide which is to be recovered. The basic principle of extracting lanthanum oxide from the monazite of the Momeik Myitsone Area.
The extraction of lanthanum from monazite follows three mains steps,
(1) By using caustic soda, the extraction of lanthanum hydroxide from monazite
(2) Digestion with nitric acid, and
(3) Precipitation with ammonium hydroxide and calcinations of lanthanum oxalate to lanthanum oxide
Experimental procedure:
Monazite sand is a raw material for this experimental procedure. Monazite to obtain 325 meshes which are finely ground and beach colour. The flow diagram of the extraction of lanthanum oxide is shown below.

Note: Individual lanthanides are applied to separate by physical method. The anhydrous fluorides and chlorides are heated under an atmosphere of argon in presence of calcium at 1270K to get individual lanthanum metal. The pure metals are obtained by heating the trifluorides of lanthanides in the presence of calcium and lithium.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE




