
What are I, II, III and IV?
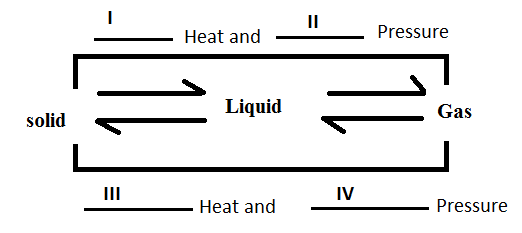
a.) Increase, Increase, Decrease, Decrease
b.) Decrease, Decrease Increase, Increase
c.) Increase, Decrease, Increase, Decrease
d.) Decrease, Increase, Decrease, Increase

Answer
592.2k+ views
Hint: The state of matter in which materials retain their boundaries without support, the atoms or molecules occupying fixed positions with respect to each other and are unable to move freely, they possess high forces of attraction and low kinetic energy.
Gas is a state of matter that has no fixed shape and no fixed volume. Gases have lower density than other states of matter, such as solids and liquids, they possess very low forces of attraction and high kinetic energy.
Complete answer:
The forces of attraction are maximum in the solid state then in liquid and are least in the gaseous state. The energy of the particles decreases as we move from solid to gaseous state as the particles in solid state are packed and have no kinetic energy to move around whereas particles in gaseous phase are free and possess high kinetic energy.
So, the energy or heat in the particles decrease as we move from solid state to gaseous state and pressure also decrease as we move from solid state to gaseous state.
So, the correct answer is “Option B”.
Note: A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a constant volume independent of pressure. As such, it is one of the four fundamental states of matter, and is the only state with a definite volume but no fixed shape; they possess intermediate forces of attraction and kinetic energy.
Gas is a state of matter that has no fixed shape and no fixed volume. Gases have lower density than other states of matter, such as solids and liquids, they possess very low forces of attraction and high kinetic energy.
Complete answer:
The forces of attraction are maximum in the solid state then in liquid and are least in the gaseous state. The energy of the particles decreases as we move from solid to gaseous state as the particles in solid state are packed and have no kinetic energy to move around whereas particles in gaseous phase are free and possess high kinetic energy.
So, the energy or heat in the particles decrease as we move from solid state to gaseous state and pressure also decrease as we move from solid state to gaseous state.
So, the correct answer is “Option B”.
Note: A liquid is a nearly incompressible fluid that conforms to the shape of its container but retains a constant volume independent of pressure. As such, it is one of the four fundamental states of matter, and is the only state with a definite volume but no fixed shape; they possess intermediate forces of attraction and kinetic energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




