
What are heterocyclic compounds?
Answer
570.6k+ views
Hint: Cyclic compounds are those compounds in which the ring atoms are made up of carbon and some other element. The atom of another element is called a heteroatom.
Complete answer:
Cyclic organic compounds are classified either as carbocycles or as heterocycles. Carbocyclic rings contain only carbon atoms whereas heterocyclic rings contain one or more different atoms along with carbon atoms. The most common heteroatoms are nitrogen, oxygen and sulphur. Heterocyclic compounds are known by their common names. For naming their derivatives, the heteroatom is always numbered as 1. We have both 5-membered (pyrrole, furan, thiophene) and 6-membered (pyridine) heterocyclic compounds.
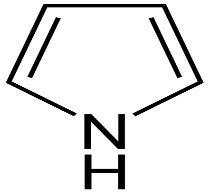
Pyrrole: It is a colourless liquid, slightly soluble in water but completely miscible with ether or ethanol. It is aromatic and is more reactive than benzene. Electrophilic substitution reaction occurs at 2-position.
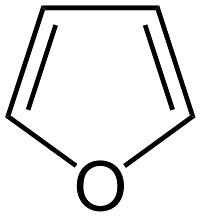
Furan: colourless liquid having odour of chloroform. It is soluble in most of the organic solvents but insoluble in ether. Furan like pyrrole undergoes electrophilic substitution at 2-position. Used as a selective solvent in petroleum refining.
Thiophene: colourless liquid having odour similar to that of benzene. It is insoluble in water but freely soluble in ether, ethanol. Thiophene is much more reactive than benzene and undergoes electrophilic
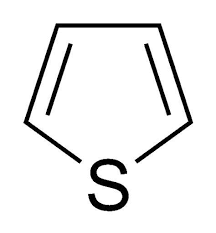
substitution reaction at 2-position under moderate conditions.
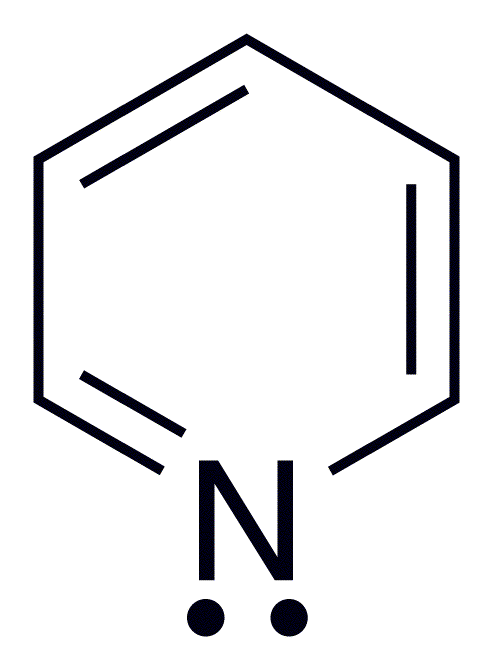
Pyridine: colourless liquid having unpleasant odour. It is soluble in water and in organic solvents. Pyridine undergoes electrophilic substitution reaction at 3-position under vigorous conditions. It is basic in nature and reacts with acids to form salts. It also undergoes nucleophilic substitution reaction at 2-position.
Note:All ring atoms in pyrrole, furan, thiophene and pyridine is $s{p^2} $ hybridised. Pyridine is less reactive towards electrophilic substitution than benzene.
Complete answer:
Cyclic organic compounds are classified either as carbocycles or as heterocycles. Carbocyclic rings contain only carbon atoms whereas heterocyclic rings contain one or more different atoms along with carbon atoms. The most common heteroatoms are nitrogen, oxygen and sulphur. Heterocyclic compounds are known by their common names. For naming their derivatives, the heteroatom is always numbered as 1. We have both 5-membered (pyrrole, furan, thiophene) and 6-membered (pyridine) heterocyclic compounds.
Pyrrole: It is a colourless liquid, slightly soluble in water but completely miscible with ether or ethanol. It is aromatic and is more reactive than benzene. Electrophilic substitution reaction occurs at 2-position.

Furan: colourless liquid having odour of chloroform. It is soluble in most of the organic solvents but insoluble in ether. Furan like pyrrole undergoes electrophilic substitution at 2-position. Used as a selective solvent in petroleum refining.

Thiophene: colourless liquid having odour similar to that of benzene. It is insoluble in water but freely soluble in ether, ethanol. Thiophene is much more reactive than benzene and undergoes electrophilic

substitution reaction at 2-position under moderate conditions.
Pyridine: colourless liquid having unpleasant odour. It is soluble in water and in organic solvents. Pyridine undergoes electrophilic substitution reaction at 3-position under vigorous conditions. It is basic in nature and reacts with acids to form salts. It also undergoes nucleophilic substitution reaction at 2-position.

Note:All ring atoms in pyrrole, furan, thiophene and pyridine is $s{p^2} $ hybridised. Pyridine is less reactive towards electrophilic substitution than benzene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




