
An organic compound $A$ of molecular formula ${C_2}{H_4}$ on reduction gives another compound $B$ of molecular formula ${C_2}{H_6}$ . $B$ on reaction with chlorine in the presence of sunlight gives $C$ of molecular formula ${C_2}{H_5}Cl$ .
Name the compounds $A,B,C$ .
Answer
569.4k+ views
Hint:
Organic compound is defined as the compound that consists of carbon and hydrogen bonds in a compound. They are covalently bonded to each other. There are around nine billion organic compounds around us.Reduction is the process in which there is an addition of hydrogen atom in a compound.
Complete step by step answer:
A.The molecular formula of compound $A$ is ${C_2}{H_4}$ . Thus the structure of the given molecular formula is as follows:
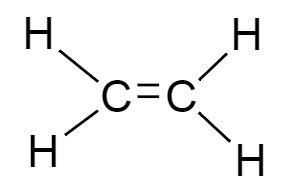 )
)
The given structure is of ethene.
Therefore, an organic compound $A$ with molecular formula ${C_2}{H_4}$is of ethene.
B.The molecular formula of compound $B$is ${C_2}{H_6}$. Thus the structure of the given molecular formula is as follows:
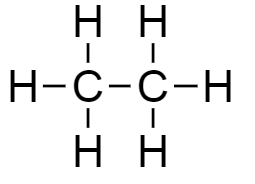
The given structure is ethane.
Therefore, an organic compound $B$ with molecular formula ${C_2}{H_6}$ is of ethane.
C.The molecular formula of compound $C$ is ${C_2}{H_5}Cl$ . Thus the structure of the given molecular formula is as follows:
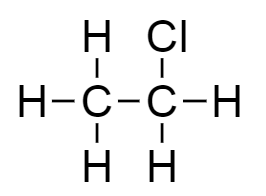
The given structure is of chloroethane.
Therefore, an organic compound $C$ with molecular formula ${C_2}{H_5}Cl$ is of chloroethane.
D.The reaction of ethane on reduction is as follows:

In this reaction ethane gets converted to ethane in the presence of $Ni/Pd$ catalyst.
During this the double bond breaks and is replaced by a hydrogen atom.
This type of reaction is known as hydrogenation.
The reaction of ethane to chloroethane is as follows
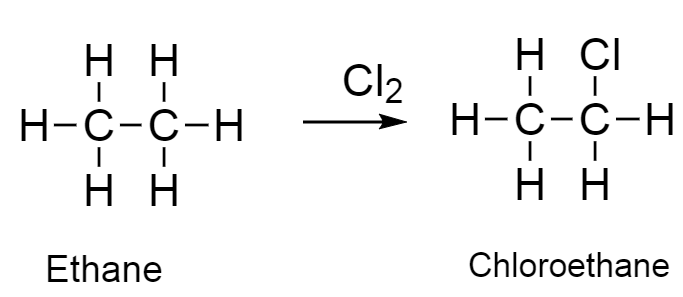
When ethane reacts with chlorine it gets converted into chloroethane as the major product and hydrochloric acid as the minor product.
This type of reaction is known as substitution reaction.
In substitution reaction hydrogen atoms get replaced by halogen such as $Cl,Br,I$ .
Alkanes undergo substitution reaction easily.
Therefore the name of the compound $A,B$ and $C$ is ethane, ethane and chloroethane respectively.
Note:Substitution reaction is defined as the reaction in which one functional group gets converted to another function groups . This type of reaction undergoes easily compounds that have single bonds. It is difficult to do a substitution reaction which consists of compounds having double or triple bonds.
Organic compound is defined as the compound that consists of carbon and hydrogen bonds in a compound. They are covalently bonded to each other. There are around nine billion organic compounds around us.Reduction is the process in which there is an addition of hydrogen atom in a compound.
Complete step by step answer:
A.The molecular formula of compound $A$ is ${C_2}{H_4}$ . Thus the structure of the given molecular formula is as follows:

The given structure is of ethene.
Therefore, an organic compound $A$ with molecular formula ${C_2}{H_4}$is of ethene.
B.The molecular formula of compound $B$is ${C_2}{H_6}$. Thus the structure of the given molecular formula is as follows:

The given structure is ethane.
Therefore, an organic compound $B$ with molecular formula ${C_2}{H_6}$ is of ethane.
C.The molecular formula of compound $C$ is ${C_2}{H_5}Cl$ . Thus the structure of the given molecular formula is as follows:

The given structure is of chloroethane.
Therefore, an organic compound $C$ with molecular formula ${C_2}{H_5}Cl$ is of chloroethane.
D.The reaction of ethane on reduction is as follows:

In this reaction ethane gets converted to ethane in the presence of $Ni/Pd$ catalyst.
During this the double bond breaks and is replaced by a hydrogen atom.
This type of reaction is known as hydrogenation.
The reaction of ethane to chloroethane is as follows

When ethane reacts with chlorine it gets converted into chloroethane as the major product and hydrochloric acid as the minor product.
This type of reaction is known as substitution reaction.
In substitution reaction hydrogen atoms get replaced by halogen such as $Cl,Br,I$ .
Alkanes undergo substitution reaction easily.
Therefore the name of the compound $A,B$ and $C$ is ethane, ethane and chloroethane respectively.
Note:Substitution reaction is defined as the reaction in which one functional group gets converted to another function groups . This type of reaction undergoes easily compounds that have single bonds. It is difficult to do a substitution reaction which consists of compounds having double or triple bonds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




