
An ideal gas is filled in compartment A of a container shown below-
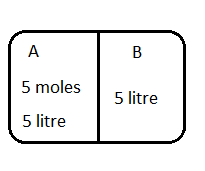
Compartment B is empty and the volume of both A and B is $5$ litre each. The partition between A and B compartments is removed suddenly. If the pressure of the gas in A was initially $4$ atm then work done by the gas during expansion is (Assuming no heat exchange with surroundings).

Answer
573.9k+ views
Hint:Gases can perform work through the expansion or compression against a constant external pressure. Work done by gases is also sometimes called pressure-volume work. When work is done on the gas, the volume of the gas further increases, and the work done is positive.
Complete step by step answer:
Since there is no heat exchange with the surroundings, it means it is an adiabatic process,
Work done$ = p\Delta V$
Pressure–volume work (or $PV$work) occurs when the volume V of the system changes. Pressure–volume work is usually measured in units of litre-atmospheres.
PV work is an important topic in chemical thermodynamics
Given,
$P = 4atm$
The initial volume, ${V_1} = 5$L
After removing the partition, the final volume is ${V_2} = 5 + 5 = 10$L
Work done$ = p\Delta V$
$
= p({V_2} - {V_1}) \\
= 4atm(10 - 5)L \\
= 20atmL \\
$
Note:
-Work is the energy required to maneuver something against a force.
-The energy of a system can change thanks to work or other kinds of energy transfer like heat. Negative work occurs when a system does work in the environment. When the gas does work, the amount of gas increases, and therefore the work done is negative.
-When a system does work on the environment, the system's internal energy decreases. When a system has work done thereon, the interior energy of the system increases. Like the heat, the energy change from work always occurs as a part of a process, as in a system can work, but doesn't contain work.
-When the gas expands against an external pressure, the gas needs to transfer some energy to the environment. Thus, the negative work decreases the energy of the gas. When the gas is compressed, energy is transferred to the gas therefore the energy of the gas increases because of positive work.
Complete step by step answer:
Since there is no heat exchange with the surroundings, it means it is an adiabatic process,
Work done$ = p\Delta V$
Pressure–volume work (or $PV$work) occurs when the volume V of the system changes. Pressure–volume work is usually measured in units of litre-atmospheres.
PV work is an important topic in chemical thermodynamics
Given,
$P = 4atm$
The initial volume, ${V_1} = 5$L
After removing the partition, the final volume is ${V_2} = 5 + 5 = 10$L
Work done$ = p\Delta V$
$
= p({V_2} - {V_1}) \\
= 4atm(10 - 5)L \\
= 20atmL \\
$
Note:
-Work is the energy required to maneuver something against a force.
-The energy of a system can change thanks to work or other kinds of energy transfer like heat. Negative work occurs when a system does work in the environment. When the gas does work, the amount of gas increases, and therefore the work done is negative.
-When a system does work on the environment, the system's internal energy decreases. When a system has work done thereon, the interior energy of the system increases. Like the heat, the energy change from work always occurs as a part of a process, as in a system can work, but doesn't contain work.
-When the gas expands against an external pressure, the gas needs to transfer some energy to the environment. Thus, the negative work decreases the energy of the gas. When the gas is compressed, energy is transferred to the gas therefore the energy of the gas increases because of positive work.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




