
An ice cube has a glass ball inside it. The cube floats on the surface of water which is kept on a trough. What happens to the water level when the ice cube melts?
Option A: remains the same.
Option B: it falls.
Option C: it rises.
Option D: It rises and falls.
Answer
582.9k+ views
Hint:Volume of water after it freezes and becomes ice is the same as the volume of water before freezing. The water molecules come close to each other and form the lattice while melting the go away from each other and break the lattice.
Complete solution:
First we have to understand the situation. There is a glass ball inside an ice cube. This ice cube is put in the water and it floats on the surface. All these are in trough.
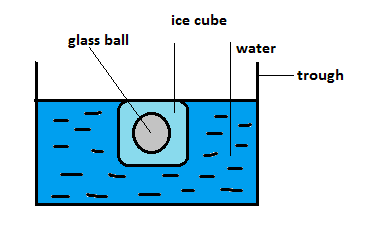
The above diagram shows the condition provided. Now you may think that the ice cube should sink and not float. This is because of a reason. The density of ice is less than that of water so it is not sinking. As the ball is stuck inside it the ball is not sinking.
Now if we see carefully the weight of the ice cube and glass ball together is balanced by the buoyant force acting on the body in upward direction.
So the ice cube with the glass ball inside it floats on the surface of the water.
Now if the ice melts, the volume now will be equal to the initial volume. As there is no considerable amount of water leaving or entering the trough.
So the total volume of the trough is not changed. Since the volume of water in solid state and that in liquid state is the same.
The glass ball’s volume was also included initially and so the net volume of the system is also not changed.
Thus option A is correct.
Note:If the cube was floating on the surface of water (with its lower face in contact with the surface) then the volume of the cube would have made a difference.
The net volume in that case would have changed and the water level would have increased.
Complete solution:
First we have to understand the situation. There is a glass ball inside an ice cube. This ice cube is put in the water and it floats on the surface. All these are in trough.

The above diagram shows the condition provided. Now you may think that the ice cube should sink and not float. This is because of a reason. The density of ice is less than that of water so it is not sinking. As the ball is stuck inside it the ball is not sinking.
Now if we see carefully the weight of the ice cube and glass ball together is balanced by the buoyant force acting on the body in upward direction.
So the ice cube with the glass ball inside it floats on the surface of the water.
Now if the ice melts, the volume now will be equal to the initial volume. As there is no considerable amount of water leaving or entering the trough.
So the total volume of the trough is not changed. Since the volume of water in solid state and that in liquid state is the same.
The glass ball’s volume was also included initially and so the net volume of the system is also not changed.
Thus option A is correct.
Note:If the cube was floating on the surface of water (with its lower face in contact with the surface) then the volume of the cube would have made a difference.
The net volume in that case would have changed and the water level would have increased.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




