
An azeotropic mixture of HCL and water has –
(A) 84% of HCI
(B) 22.2% HCI
(C) 63% of HCI
(D) 20.2% HCI
Answer
596.1k+ views
Hint: An azeotropic mixture of HCL and water is a negative boiling azeotrope. It can also be called a maximum boiling azeotrope. It has more concentration of HCl than water.
Complete step by step solution:
- Azeotropes are mixtures of two or more liquids that have the same concentration in both vapour phase and liquid phase.
- This means that when an azeotrope is boiled, the vapour of the constituents will have the same proportion of the constituents as the unboiled mixture.
- Because of this property of azeotropes, their composition will be unchanged upon simple distillation and hence this method cannot be used for separating the components.
- They are also called as constant boiling point mixtures as the mixture boils at a constant temperature.
- Azeotropes do not follow Raoult’s law (${{P}_{A}}={{P}_{A}}^{O}\times {{X}_{A}}$ ).
- Azeotropes are classified depending on the type of deviation from Raoult’s law.
- A solution showing negative deviation from Raoult’s law is called a maximum boiling azeotrope.
- A solution showing positive deviation from Raoult’s law is called a minimum boiling azeotrope.
- Azeotropes can be classified into positive and negative azeotropes on the basis of the characteristic boiling point of the azeotrope.
- The characteristic boiling point of an azeotrope will be either lesser or greater than the boiling point of any of its constituents.
- If the boiling point of the azeotropic mixture is lesser than the boiling point of any of its constituents, it is said to be a positive azeotrope.
- If the boiling point of the azeotropic mixture is greater than the boiling point of any of its constituents, it is said to be a negative azeotrope.
- From these 2 classifications, we can understand that positive azeotropes are minimum boiling azeotropes and negative azeotropes are maximum boiling azeotropes.
- The positive and negative azeotropes can be represented in a graph as-
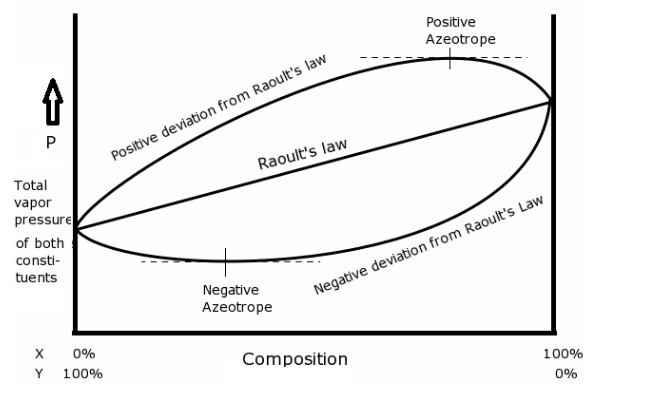
- The azeotrope given in the question is a mixture of HCl and water. This is an example of a negative azeotrope.
- This azeotrope has a concentration of 20.2% hydrochloric acid and 79.8% water (by mass).
-Hence, the answer is option (D) 20.2% HCI.
Note: Azeotropes can also be classified on the basis of the miscibility of its components. A homogeneous azeotrope is one in which the constituents of the mixture are completely miscible in all proportions. A heterogeneous azeotrope is one in which the constituents of the mixture are not completely miscible.
Complete step by step solution:
- Azeotropes are mixtures of two or more liquids that have the same concentration in both vapour phase and liquid phase.
- This means that when an azeotrope is boiled, the vapour of the constituents will have the same proportion of the constituents as the unboiled mixture.
- Because of this property of azeotropes, their composition will be unchanged upon simple distillation and hence this method cannot be used for separating the components.
- They are also called as constant boiling point mixtures as the mixture boils at a constant temperature.
- Azeotropes do not follow Raoult’s law (${{P}_{A}}={{P}_{A}}^{O}\times {{X}_{A}}$ ).
- Azeotropes are classified depending on the type of deviation from Raoult’s law.
- A solution showing negative deviation from Raoult’s law is called a maximum boiling azeotrope.
- A solution showing positive deviation from Raoult’s law is called a minimum boiling azeotrope.
- Azeotropes can be classified into positive and negative azeotropes on the basis of the characteristic boiling point of the azeotrope.
- The characteristic boiling point of an azeotrope will be either lesser or greater than the boiling point of any of its constituents.
- If the boiling point of the azeotropic mixture is lesser than the boiling point of any of its constituents, it is said to be a positive azeotrope.
- If the boiling point of the azeotropic mixture is greater than the boiling point of any of its constituents, it is said to be a negative azeotrope.
- From these 2 classifications, we can understand that positive azeotropes are minimum boiling azeotropes and negative azeotropes are maximum boiling azeotropes.
- The positive and negative azeotropes can be represented in a graph as-

- The azeotrope given in the question is a mixture of HCl and water. This is an example of a negative azeotrope.
- This azeotrope has a concentration of 20.2% hydrochloric acid and 79.8% water (by mass).
-Hence, the answer is option (D) 20.2% HCI.
Note: Azeotropes can also be classified on the basis of the miscibility of its components. A homogeneous azeotrope is one in which the constituents of the mixture are completely miscible in all proportions. A heterogeneous azeotrope is one in which the constituents of the mixture are not completely miscible.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




