
An aromatic compound of molecular formula \[C_6H_4Br_2\] was nitrated then only one product of formula \[C_6H_3Br_2NO_2\] was obtained. The original compound is:
A.O-dibromobenzene
B.M-dibromobenzene
C.P-dibromobenzene
D.Both A and C
Answer
573.9k+ views
Hint:Halogens like bromine, are electron withdrawing groups which deactivates the ring, and it also shows inductive effect.
-The inductive effect overpowers the resonance effect, so it ultimately becomes ortho para directing, to the electrophilic substitution reaction.
Complete answer:
-Halogens have the tendency of withdrawing electrons due to high electronegativity and electron affinity. They have a very high inductive effect and electron withdrawing power. In electrophilic substitution reactions it shows both electron donating resonance effect and electron withdrawing inductive effect, but the inductive effect overpowers resonance effect so it deactivates benzene ring and directs it to ortho para positions.
-It is given that the reactant should have such a structure, which gives only one compound as a product, which means it should have symmetry in its structure.
-The two bromine groups are attached to para position to each other in p-dibromobenzene. Nitration of p-dibromobenzene gives only one product that is \[1,4-dibromo-2-nitrobenzene\], this is because, no matter where the nitro group gets attached it will always give the same compound. In other words the four attacking sites are identical because of the presence of two bromine groups at the para position to each other. This reaction can be represented as,
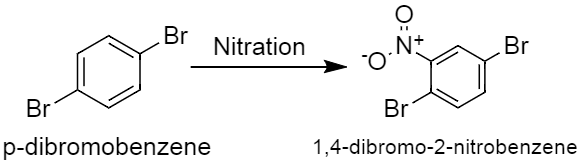
The other option given in the question, where the bromine groups are in meta position to each other, nitration of that compound will at least give two products. One of them will be major and the other one will be a minor product. In case of o-dibromobenzene, the bromine groups are in ortho position to each other, which will also give at least two products, if they undergo the process of nitration.
As we can see, the only appropriate option which is correct, is option C, as it gives only one product which matches the description given in the question.
So the correct answer is, option C.
Note:
-Bromine is an electron withdrawing groups, because of presence of seven electrons in its outermost shell
-It needs only one electron to complete its octet, and to become stable, that is why it is electronegative in nature
-The inductive effect overpowers the resonance effect, so it ultimately becomes ortho para directing, to the electrophilic substitution reaction.
Complete answer:
-Halogens have the tendency of withdrawing electrons due to high electronegativity and electron affinity. They have a very high inductive effect and electron withdrawing power. In electrophilic substitution reactions it shows both electron donating resonance effect and electron withdrawing inductive effect, but the inductive effect overpowers resonance effect so it deactivates benzene ring and directs it to ortho para positions.
-It is given that the reactant should have such a structure, which gives only one compound as a product, which means it should have symmetry in its structure.
-The two bromine groups are attached to para position to each other in p-dibromobenzene. Nitration of p-dibromobenzene gives only one product that is \[1,4-dibromo-2-nitrobenzene\], this is because, no matter where the nitro group gets attached it will always give the same compound. In other words the four attacking sites are identical because of the presence of two bromine groups at the para position to each other. This reaction can be represented as,

The other option given in the question, where the bromine groups are in meta position to each other, nitration of that compound will at least give two products. One of them will be major and the other one will be a minor product. In case of o-dibromobenzene, the bromine groups are in ortho position to each other, which will also give at least two products, if they undergo the process of nitration.
As we can see, the only appropriate option which is correct, is option C, as it gives only one product which matches the description given in the question.
So the correct answer is, option C.
Note:
-Bromine is an electron withdrawing groups, because of presence of seven electrons in its outermost shell
-It needs only one electron to complete its octet, and to become stable, that is why it is electronegative in nature
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




