
An alkyl halide may be converted into alcohol by ____ reaction
A.Electrophilic substitution
B.Elimination reaction
C.Nucleophilic substitution
D.None of these
Answer
571.5k+ views
Hint: We have to remember that the alkyl halides (aka haloalkanes or halogenoalkanes) contain one or more carbon atoms. General formula is \[R - X\].
Where, $R - $alkyl group or a substituted group & $X - $halogen atom $(F,Cl,Br,I)$.
It is derived from alkanes. It is a subset of the general class of halocarbons. The most important reactions of alkyl halide is substitution and elimination.
Complete step by step solution:
We have to know that the alkyl halides, in an aliphatic hydrocarbon the hydrogen atoms are replaced by halogen atoms. Alkyl halides generally undergo substitution and elimination types of organic reactions.
As we know, nucleophilic Substitution reaction is a type of chemical reaction in which one functional group is replaced by another functional group. In a nucleophilic substitution reaction, an electron rich nucleophile is replaced by a positively charged electrophile.
In a halide, the carbon bonded to a halide, called alpha$(\alpha )$-carbon and the beta$(\beta )$-carbon is bonded to the alpha-carbon and also continuing $(\alpha ,\beta ,\gamma ,\delta ,etc)$ carbons. For example $2 - chloropropane$.
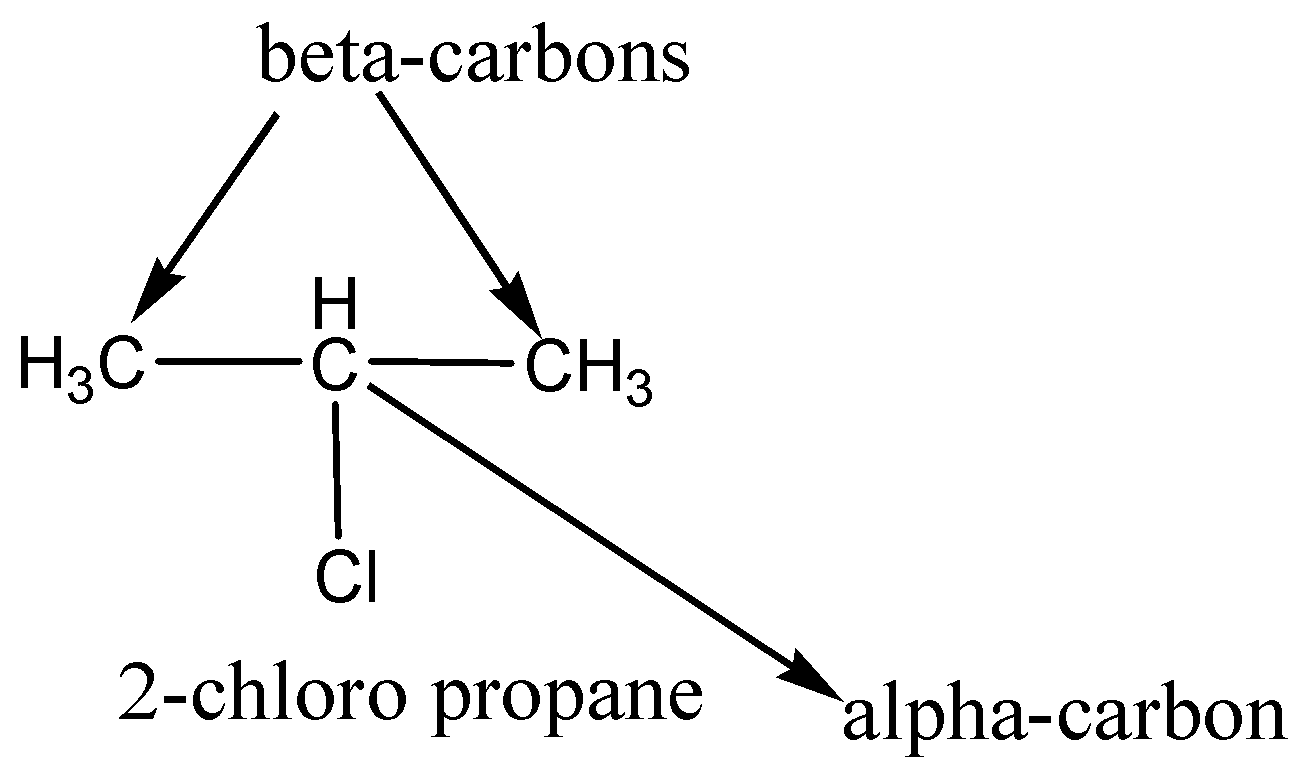
We have to remember that alkyl halides undergo a reaction called a nucleophilic substitution reaction, because the alkyl halide contains electrophilic groups that bond with a nucleophile, replacing (substitutes) the halogen. The halogen is left and so called the leaving group. In alkyl halides the halogens share a bond with carbon (they are polarizable) and are excellent electrophiles.
For example,$2 - chloropropane$ is converted into \[{\text{propan - 2 - ol}}\] in a substitution reaction.
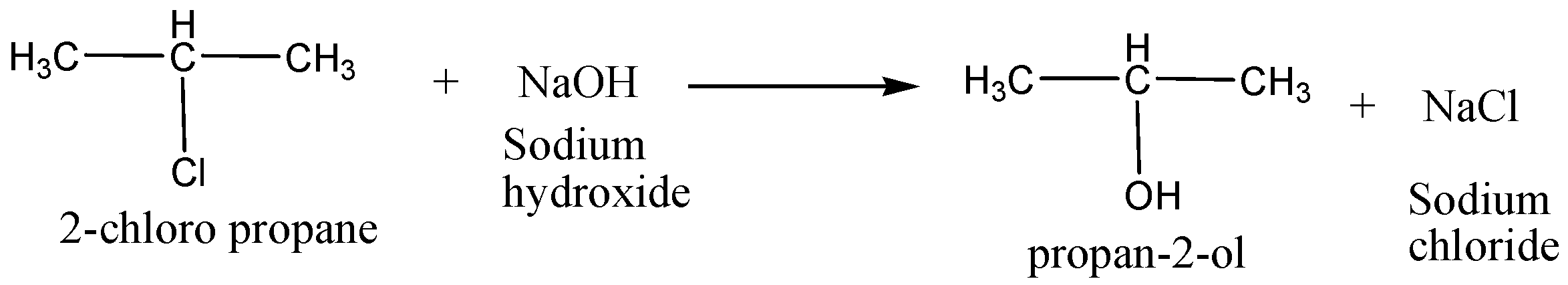
In the above chemical reaction, alkyl halide ($2 - chloropropane$), contains electrophile (bond with nucleophilic carbon) replacing a halogen by a nucleophile to give corresponding alcohol.
In an elimination reaction the alkyl halide reacted with a base to produce a $C = C$ to form an alkene. See the below example,
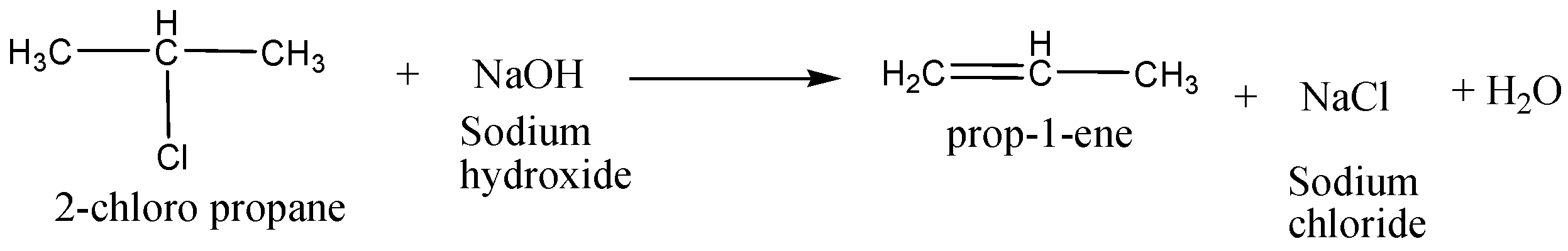
Alkyl halide ($2 - chloropropane$) reacted with a base to give an alkene $(prop - 2 - ene)$.
So, from the above information an alkyl halide may be converted into alcohol by nucleophilic substitution reaction.
Therefore, the option (c) Nucleophilic substitution is the correct answer.
Note:We have to remember that the elimination reaction is a chemical reaction, a pair of atoms or molecules are removed from a molecule.
As we know that electrophilic Substitution reaction is a type of chemical reaction in which one functional group is replaced by another functional group. In an electrophilic substitution reaction, an electrophile replaces a functional group attached to a compound.
Where, $R - $alkyl group or a substituted group & $X - $halogen atom $(F,Cl,Br,I)$.
It is derived from alkanes. It is a subset of the general class of halocarbons. The most important reactions of alkyl halide is substitution and elimination.
Complete step by step solution:
We have to know that the alkyl halides, in an aliphatic hydrocarbon the hydrogen atoms are replaced by halogen atoms. Alkyl halides generally undergo substitution and elimination types of organic reactions.
As we know, nucleophilic Substitution reaction is a type of chemical reaction in which one functional group is replaced by another functional group. In a nucleophilic substitution reaction, an electron rich nucleophile is replaced by a positively charged electrophile.
In a halide, the carbon bonded to a halide, called alpha$(\alpha )$-carbon and the beta$(\beta )$-carbon is bonded to the alpha-carbon and also continuing $(\alpha ,\beta ,\gamma ,\delta ,etc)$ carbons. For example $2 - chloropropane$.

We have to remember that alkyl halides undergo a reaction called a nucleophilic substitution reaction, because the alkyl halide contains electrophilic groups that bond with a nucleophile, replacing (substitutes) the halogen. The halogen is left and so called the leaving group. In alkyl halides the halogens share a bond with carbon (they are polarizable) and are excellent electrophiles.
For example,$2 - chloropropane$ is converted into \[{\text{propan - 2 - ol}}\] in a substitution reaction.

In the above chemical reaction, alkyl halide ($2 - chloropropane$), contains electrophile (bond with nucleophilic carbon) replacing a halogen by a nucleophile to give corresponding alcohol.
In an elimination reaction the alkyl halide reacted with a base to produce a $C = C$ to form an alkene. See the below example,

Alkyl halide ($2 - chloropropane$) reacted with a base to give an alkene $(prop - 2 - ene)$.
So, from the above information an alkyl halide may be converted into alcohol by nucleophilic substitution reaction.
Therefore, the option (c) Nucleophilic substitution is the correct answer.
Note:We have to remember that the elimination reaction is a chemical reaction, a pair of atoms or molecules are removed from a molecule.
As we know that electrophilic Substitution reaction is a type of chemical reaction in which one functional group is replaced by another functional group. In an electrophilic substitution reaction, an electrophile replaces a functional group attached to a compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




