
An aldohexose can exist in $16$ isomeric forms.
A: True
B: False
Answer
591k+ views
Hint: Isomers are the chemical compounds that have the same molecular formula but arrangement of atoms is different. Isomers are of many types. Aldohexose is a hexose which has an aldehyde group on one end. Hexose is a monosaccharide with six carbon atoms.
Complete step by step answer:
Isomers are the chemical species which have the same molecular formula but different structures. Aldohexose is a chemical compound in which there is an aldehyde group at one end of hexose. Hexose is a monosaccharide with six carbon atoms. Monosaccharide is the simple sugar. General formula of monosaccharide is ${\left( {C{H_2}O} \right)_n}$. Aldehyde is a fuctional group with chemical formula $ - OH$. Chemical formula of aldohexose is $H - C\left( { = O} \right) - {\left( {CHOH} \right)_5} - H$. Glucose is the most common example of aldohexose. Glucose is made by plants during photosynthesis from water and carbon dioxide taking energy from sunlight. Glucose is the subcategory of carbohydrates. Isomers of aldohexose are as follows:
$1.$ $D - $Allose
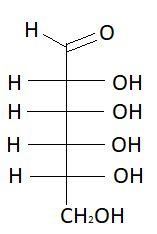
$2.$ $L - $Allose
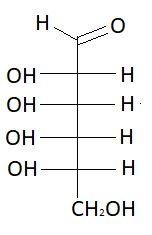
$3.$ $D - $Altrose
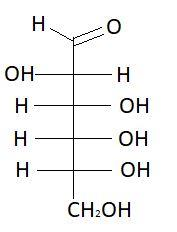
$4.$ $L - $ Altrose
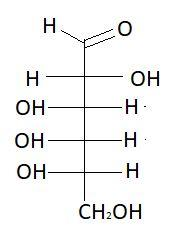
$5.$ $D - $Glucose
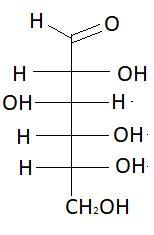
$6.$ $L - $ Glucose
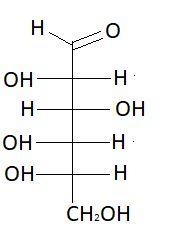
$7.$ $D - $Mannose
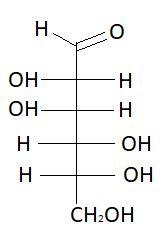
$8.$ $L - $ Mannose
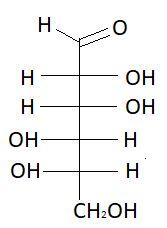
$9.$ $D - $Gulose
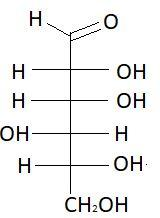
$10.$ $L - $ Gulose
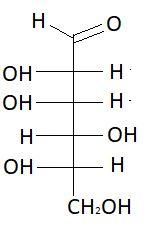
$11.$ $D - $Idose
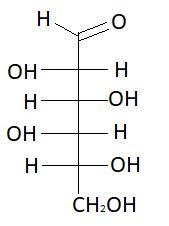
$12.$ $L - $ Idose
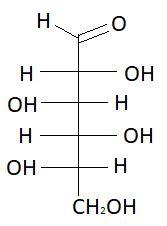
$13.$ $D - $Galactose
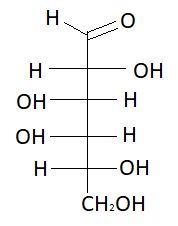
$14.$ $L - $ Galactose
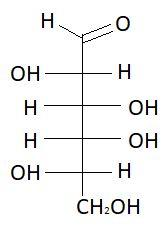
$15.$ $D - $Talose
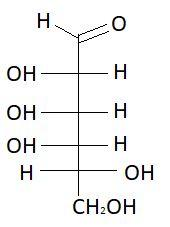
$16.$ $L - $Talose
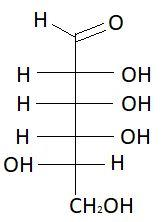
These are the isomers of aldohexose. There are total $16$ isomers of aldohexose. The statement states that an aldohexose can exist in $16$ isomeric forms, which is true.
Note:
In the name of the isomers of aldohexose, the terms $L$ and $D$ are present. $D$ represents that the compound is dextrorotatory, that is the compound has the ability to rotate planes of polarized light in the right direction. $L$ represents levorotatory meaning that the compound has the ability to rotate planes polarized in the left direction.
Complete step by step answer:
Isomers are the chemical species which have the same molecular formula but different structures. Aldohexose is a chemical compound in which there is an aldehyde group at one end of hexose. Hexose is a monosaccharide with six carbon atoms. Monosaccharide is the simple sugar. General formula of monosaccharide is ${\left( {C{H_2}O} \right)_n}$. Aldehyde is a fuctional group with chemical formula $ - OH$. Chemical formula of aldohexose is $H - C\left( { = O} \right) - {\left( {CHOH} \right)_5} - H$. Glucose is the most common example of aldohexose. Glucose is made by plants during photosynthesis from water and carbon dioxide taking energy from sunlight. Glucose is the subcategory of carbohydrates. Isomers of aldohexose are as follows:
$1.$ $D - $Allose

$2.$ $L - $Allose

$3.$ $D - $Altrose

$4.$ $L - $ Altrose

$5.$ $D - $Glucose

$6.$ $L - $ Glucose

$7.$ $D - $Mannose

$8.$ $L - $ Mannose

$9.$ $D - $Gulose

$10.$ $L - $ Gulose

$11.$ $D - $Idose

$12.$ $L - $ Idose

$13.$ $D - $Galactose

$14.$ $L - $ Galactose

$15.$ $D - $Talose

$16.$ $L - $Talose

These are the isomers of aldohexose. There are total $16$ isomers of aldohexose. The statement states that an aldohexose can exist in $16$ isomeric forms, which is true.
Note:
In the name of the isomers of aldohexose, the terms $L$ and $D$ are present. $D$ represents that the compound is dextrorotatory, that is the compound has the ability to rotate planes of polarized light in the right direction. $L$ represents levorotatory meaning that the compound has the ability to rotate planes polarized in the left direction.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




