
Amongst \[N{O_3}^ - ,{\text{ }}As{O_3}^{3 - },{\text{ }}C{O_3}^{2 - },{\text{ }}Cl{O_3}^ - ,{\text{ }}S{O_3}^{2 - }\;and\;B{O_3}^{3 - }\], the non-planar species are :
\[\begin{array}{*{20}{l}}
{A:N{O_3}^ - ,{\text{ }}C{O_3}^{2 - },{\text{ }}and{\text{ }}B{O_3}^{3 - }} \\
{B:{\text{ }}S{O_3}^{2 - },{\text{ }}Cl{O_3}^ - ,{\text{ }}and{\text{ }}As{O_3}^{3 - }} \\
{C:{\text{ }}C{O_2}^{3 - },{\text{ }}S{O_3}^{2 - }{,^{}}andB{O_3}^{3 - }} \\
{D:{\text{ }}As{O_3}^{3 - },{\text{ }}C{O_3}^{2 - },{\text{ }}and{\text{ }}S{O_3}^{2 - }}
\end{array}\]
Answer
577.8k+ views
Hint:A planar molecule refers to a molecule with all its atoms lying in one two-dimensional plane. Molecules having linear, square or triangular geometries are said to be planar molecules. In other cases, molecules may form three-dimensional shapes, like tetrahedrons, bipyramids or octahedrons.
Complete step by step answer:
First of all you have to find the hybridization of molecules. You can remember following rules to decide whether a molecule is planar or non-planar:
1) The molecule is not considered to be planar If there exists a $sp_3$ hybridized carbon (or nitrogen).
2) The molecule is considered to be planar if there is no $sp_3$hybridized carbon (or nitrogen), but there is one $sp_2$ h hybridized carbon or nitrogen atom.
3) The molecule is not considered to be planar if there is no sp3 hybridized atom but there are two $sp_2$ hybridized atoms which are separated by an even number of double bonds.
We can say that the molecule is not planar if there exists either a $sp_3$ hybridized atom or two $sp_2$ hybridized atoms that are being separated by even numbers of double bonds. In other cases, its structure is planar. For the present scenario, consider the following table:
\[N{O_3}^ - ,{\text{ }}C{O_3}^{2 - } and {\text{ }}B{O_3}^{3 - }\] possess $sp_2$ hybridisation and thus, are planar species.
Hence, the correct answer is Option B.
Note:
You can remember that in order to find the hybridization of a molecule, count the valence electrons of each atom in a compound (fact to be noted is that count valence electron of hydrogen = 7). Add them all and divide the number by 8. Quotient represents bond pair while $\dfrac{{remainder}}{2}$ represents lone pair. Add quotient and $\dfrac{{remainder}}{2}$ and assume it as x. If (i) x is 3, molecule is planar with sp2 hybridisation, (ii) x is 2, molecule is linear with sp hybridization and x is 4, molecule is non-planar with sp3 hybridization.
Complete step by step answer:
First of all you have to find the hybridization of molecules. You can remember following rules to decide whether a molecule is planar or non-planar:
1) The molecule is not considered to be planar If there exists a $sp_3$ hybridized carbon (or nitrogen).
2) The molecule is considered to be planar if there is no $sp_3$hybridized carbon (or nitrogen), but there is one $sp_2$ h hybridized carbon or nitrogen atom.
3) The molecule is not considered to be planar if there is no sp3 hybridized atom but there are two $sp_2$ hybridized atoms which are separated by an even number of double bonds.
We can say that the molecule is not planar if there exists either a $sp_3$ hybridized atom or two $sp_2$ hybridized atoms that are being separated by even numbers of double bonds. In other cases, its structure is planar. For the present scenario, consider the following table:
| Species | Hybridization | Geometry | No. of lone pairs |
| NO3- | sp2 | 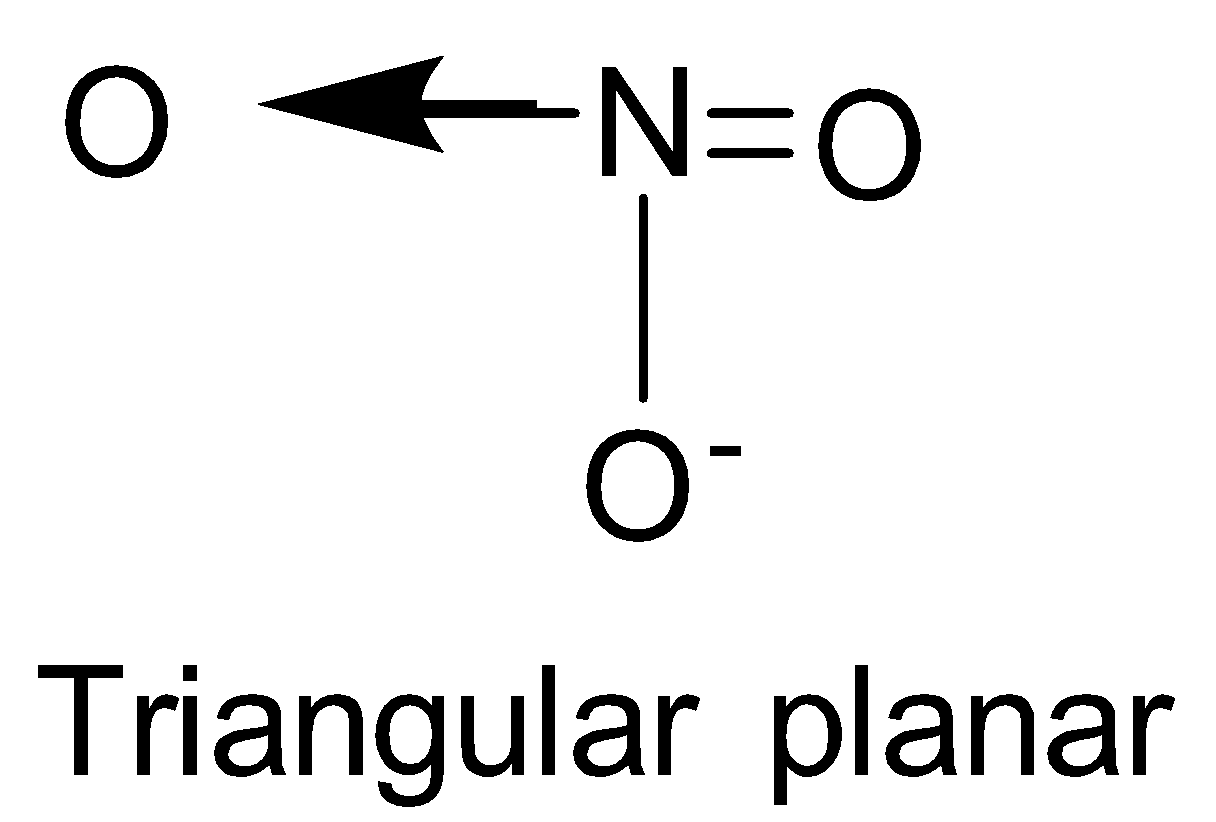
| 0 |
| AsO33- | sp3 | 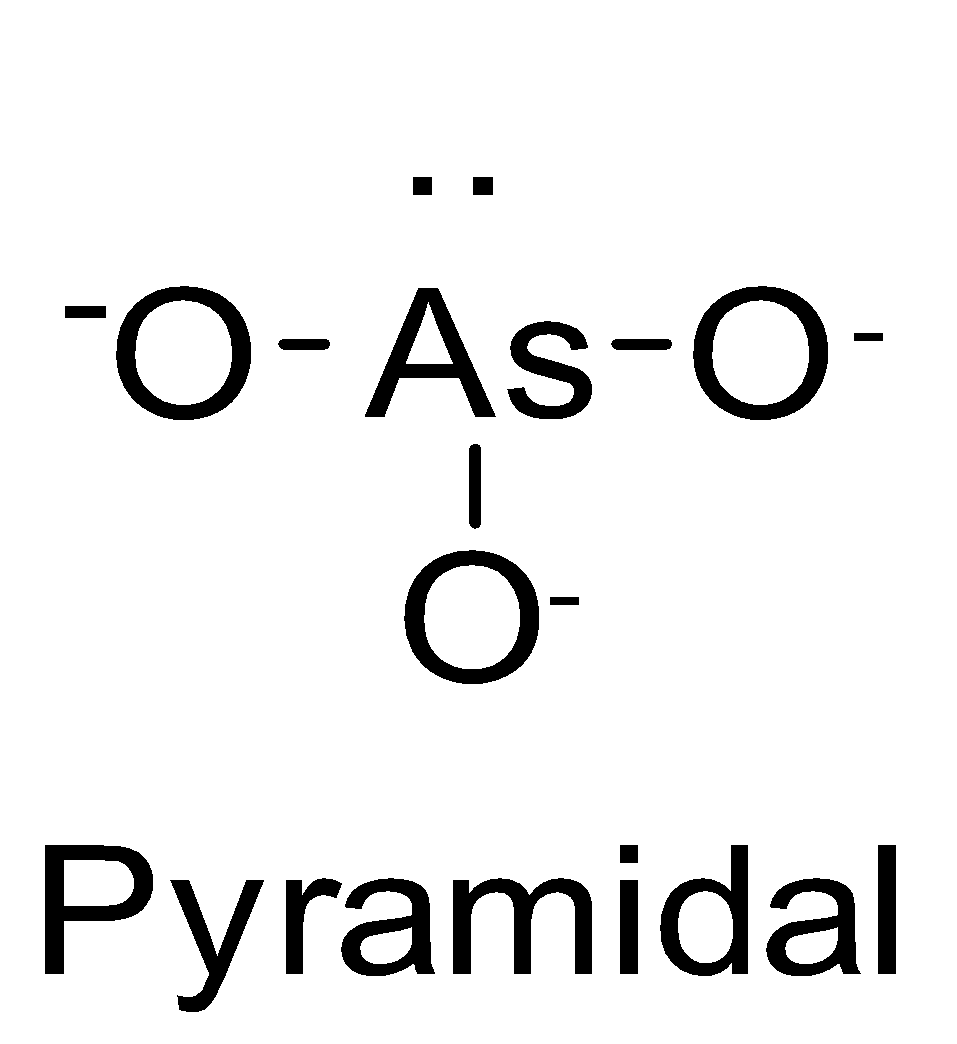
| 1 |
| CO32- | sp2 | 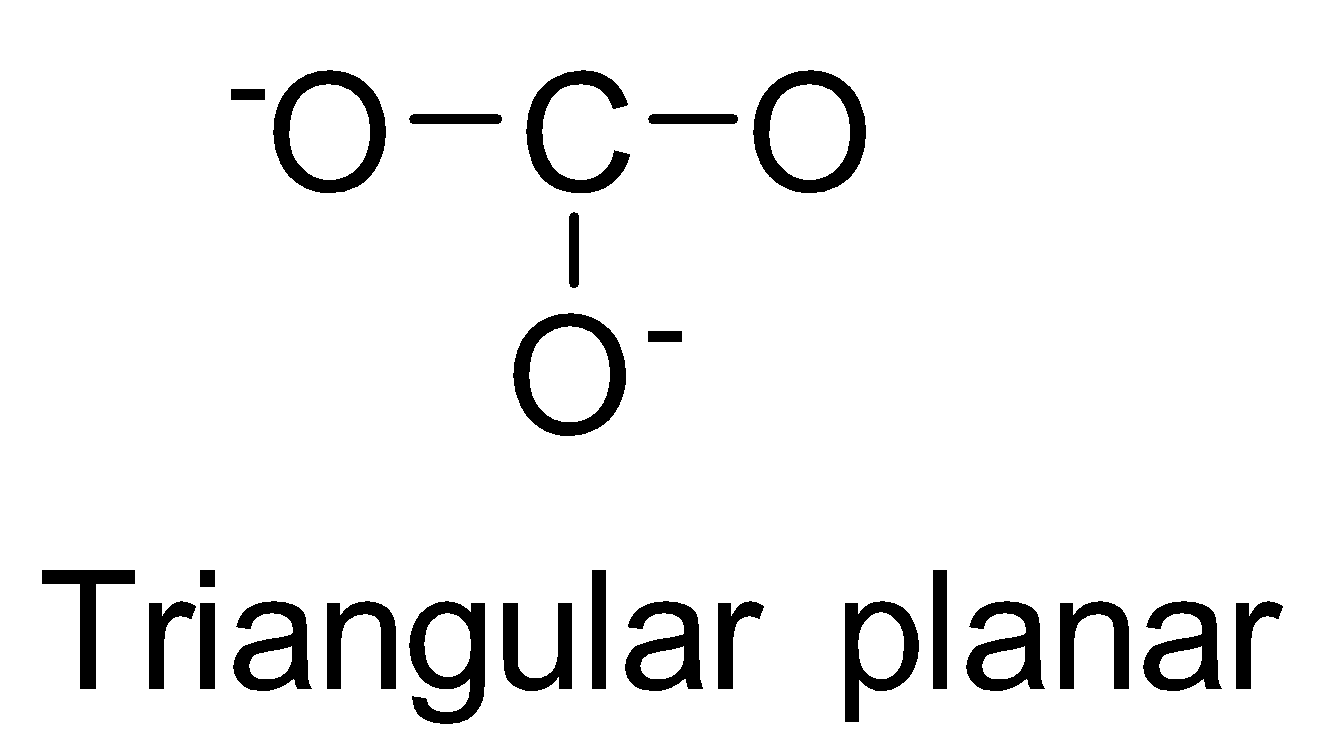
| 0 |
| ClO3- | sp3 | 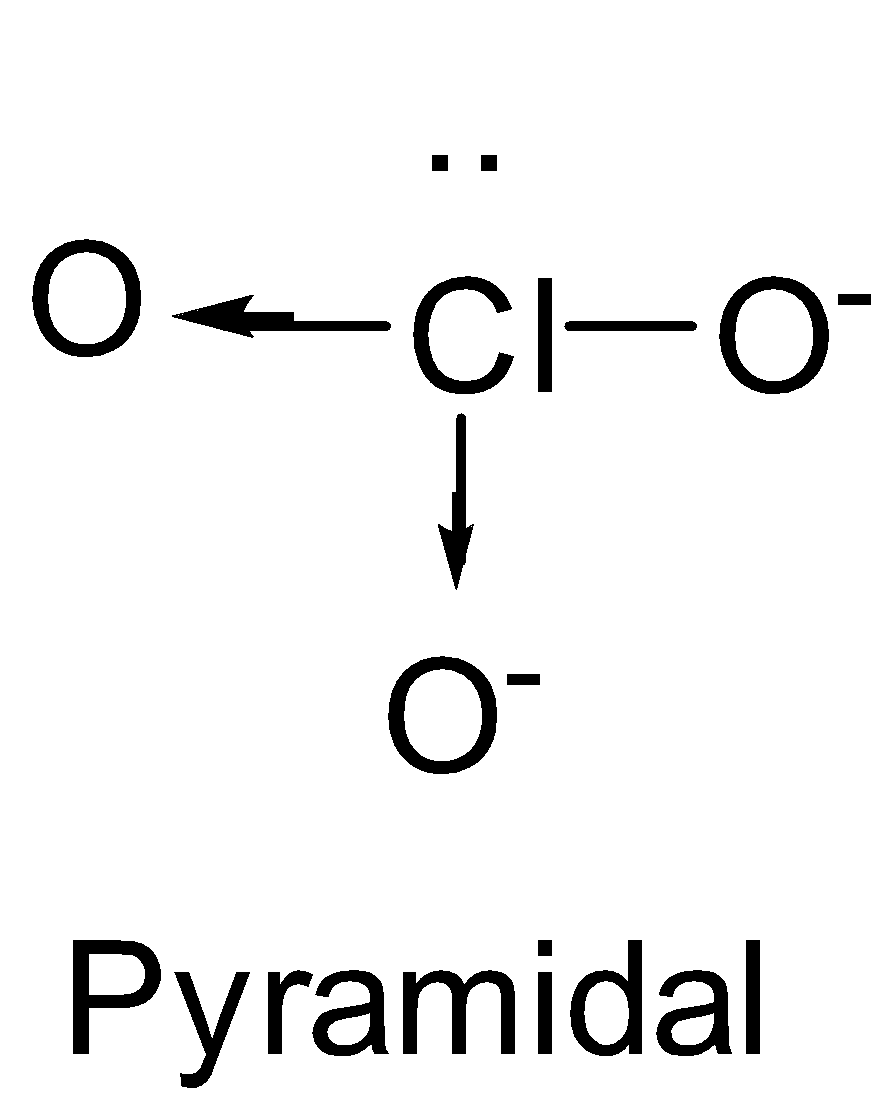
| 1 |
| SO32- | sp3 | 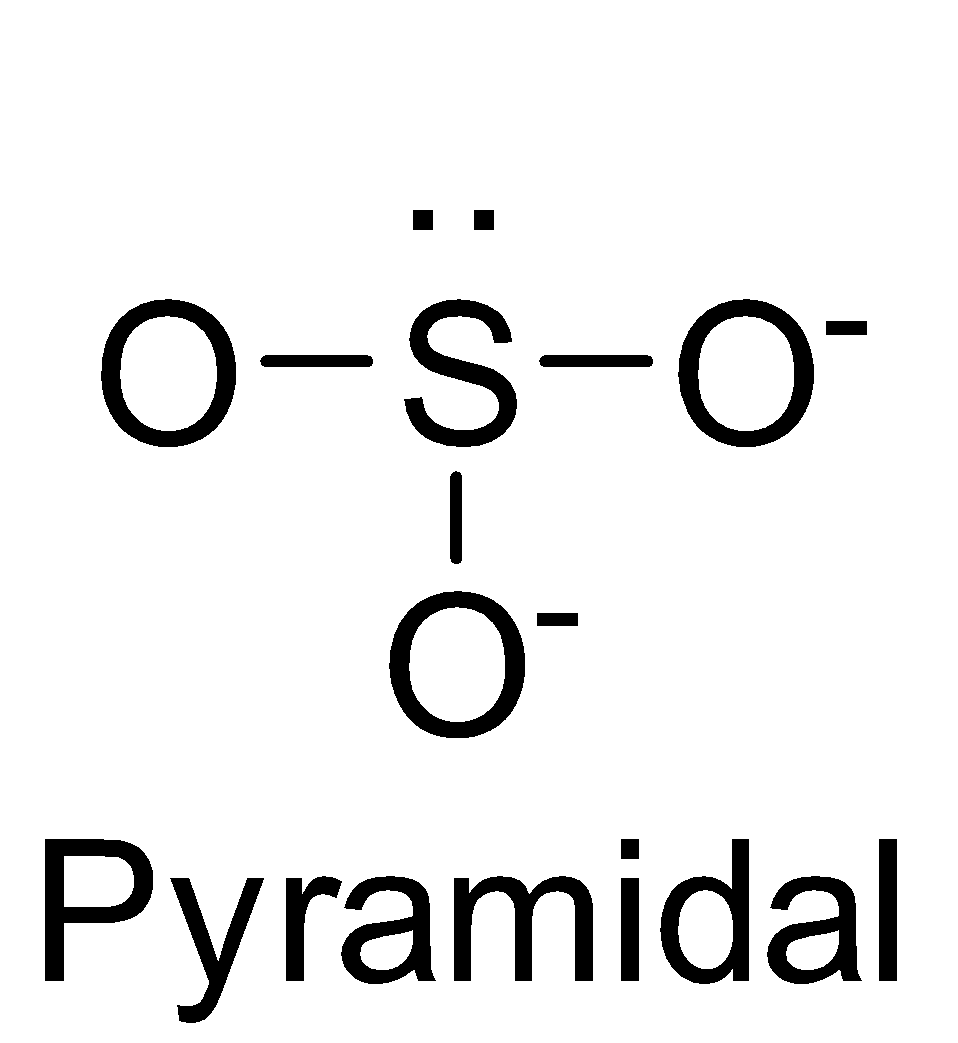
| 1 |
| BO33- | sp2 | 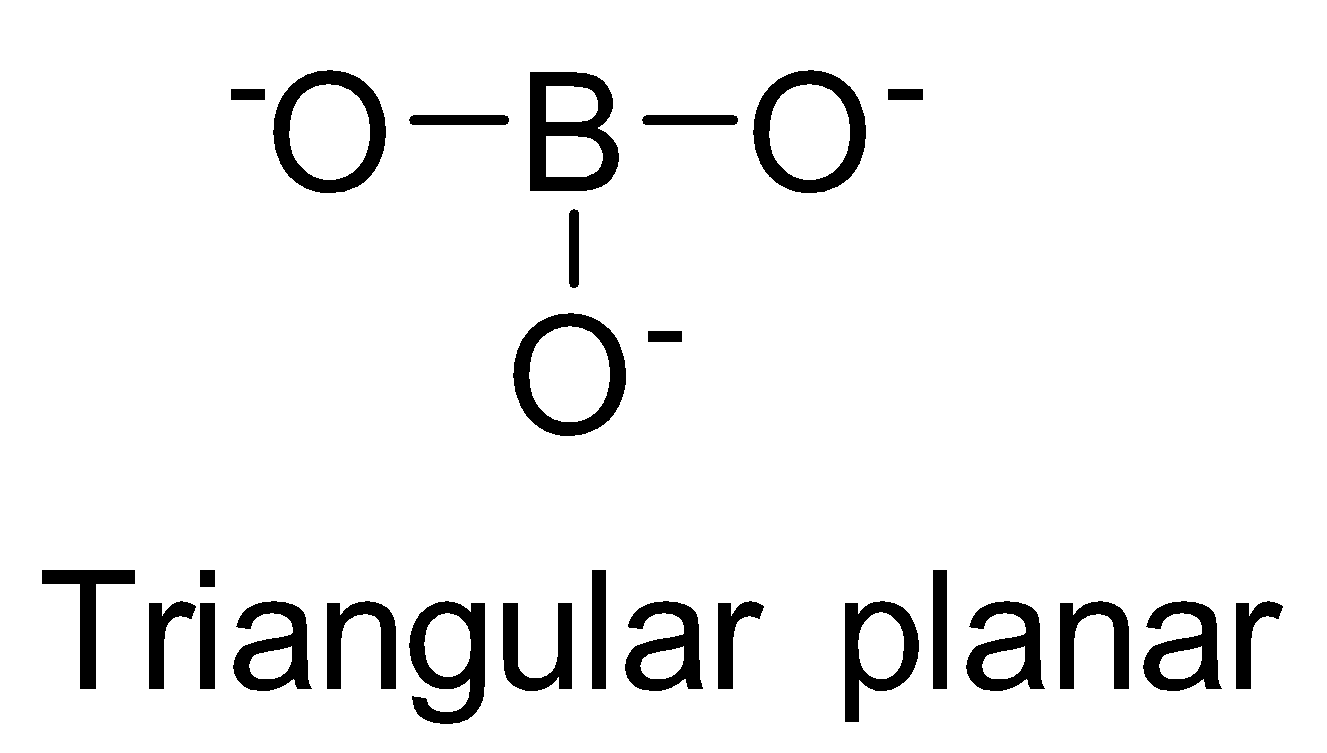
| 0 |
\[N{O_3}^ - ,{\text{ }}C{O_3}^{2 - } and {\text{ }}B{O_3}^{3 - }\] possess $sp_2$ hybridisation and thus, are planar species.
Hence, the correct answer is Option B.
Note:
You can remember that in order to find the hybridization of a molecule, count the valence electrons of each atom in a compound (fact to be noted is that count valence electron of hydrogen = 7). Add them all and divide the number by 8. Quotient represents bond pair while $\dfrac{{remainder}}{2}$ represents lone pair. Add quotient and $\dfrac{{remainder}}{2}$ and assume it as x. If (i) x is 3, molecule is planar with sp2 hybridisation, (ii) x is 2, molecule is linear with sp hybridization and x is 4, molecule is non-planar with sp3 hybridization.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




