
Among ${N_2}O,S{O_2},I_3^ + ,I_3^ - $ , the linear species are ${N_2}O$ and $I_3^ - $ .
A.True
B.False
Answer
570.3k+ views
Hint:
Linear species means the structure of the given compound is linear. It is aligned horizontally. Every molecule has different shape and structure depending upon the number of lone pairs and the type of bonding it has.
Complete step by step answer:
1.${N_2}O$
Nitrous oxide is also known as the laughing gas.
It is a non flammable gas at room temperature.
It is slightly metallic in taste and smell.
It is heavier than air.
The lewis structure of nitrous oxide is as follows:
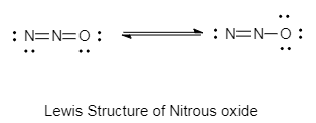
In the diagram given above, the type of bonding in nitrous oxide is covalent bonding.
Due to the presence of sigma bonds and absence of lone pairs on the central atom, the stable structure of nitrous acid is linear.
2.$S{O_2}$
It is also known as sulphur dioxide.
The sulphur dioxide gas is toxic.
The smell of the burnt match sticks is of the sulphur dioxide.
It is a covalent compound.
The structure of sulphur dioxide is as follows:
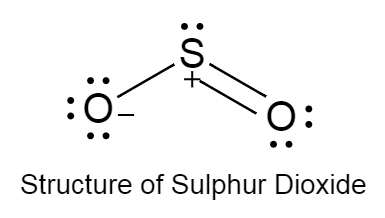
The structure of sulphur dioxide is bent because due to the high electronegativity of oxygen it tries to pull sulphur atom towards itself.
3.$I_3^ + $
The structure of $I_3^ + $ is given below as follows:
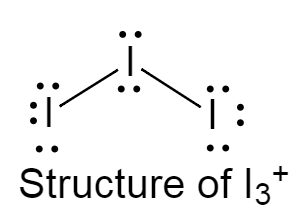
It has a bent structure because of the presence of two lone pairs of electrons on the central iodine atom.
4.$I_3^ - $
The structure of $I_3^ - $ is as follows:
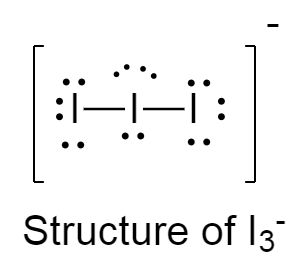
There are three lone pairs on the central iodine atom because of which the adjacent iodine atoms try to repel from each other as much as possible at a greater distance causing the angle to be ${180^ \circ }$ .
Because of this repulsion, The structure of $I_3^ - $ is linear.
Since the structure of $I_3^ - $and ${N_2}O$ is linear, they are linear species.
So the correct answer is option A) True.
Note: $I_3^ - $ is neither polar or nonpolar molecule. As it is soluble in water it is known as a polar molecule. $I_3^ - $ has more number of lone pairs as compared to $I_3^ + $ . The smell of sulphur dioxide gas is not at all tolerable.
Linear species means the structure of the given compound is linear. It is aligned horizontally. Every molecule has different shape and structure depending upon the number of lone pairs and the type of bonding it has.
Complete step by step answer:
1.${N_2}O$
Nitrous oxide is also known as the laughing gas.
It is a non flammable gas at room temperature.
It is slightly metallic in taste and smell.
It is heavier than air.
The lewis structure of nitrous oxide is as follows:

In the diagram given above, the type of bonding in nitrous oxide is covalent bonding.
Due to the presence of sigma bonds and absence of lone pairs on the central atom, the stable structure of nitrous acid is linear.
2.$S{O_2}$
It is also known as sulphur dioxide.
The sulphur dioxide gas is toxic.
The smell of the burnt match sticks is of the sulphur dioxide.
It is a covalent compound.
The structure of sulphur dioxide is as follows:

The structure of sulphur dioxide is bent because due to the high electronegativity of oxygen it tries to pull sulphur atom towards itself.
3.$I_3^ + $
The structure of $I_3^ + $ is given below as follows:

It has a bent structure because of the presence of two lone pairs of electrons on the central iodine atom.
4.$I_3^ - $
The structure of $I_3^ - $ is as follows:

There are three lone pairs on the central iodine atom because of which the adjacent iodine atoms try to repel from each other as much as possible at a greater distance causing the angle to be ${180^ \circ }$ .
Because of this repulsion, The structure of $I_3^ - $ is linear.
Since the structure of $I_3^ - $and ${N_2}O$ is linear, they are linear species.
So the correct answer is option A) True.
Note: $I_3^ - $ is neither polar or nonpolar molecule. As it is soluble in water it is known as a polar molecule. $I_3^ - $ has more number of lone pairs as compared to $I_3^ + $ . The smell of sulphur dioxide gas is not at all tolerable.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




