
Although phenoxide ion has more no. of resonating structure than carboxylate ion, the carboxylic acid is a stronger acid than phenol. Give two reasons.
Answer
597.3k+ views
Hint:
The acidic strength depends on the stability of the conjugate base which is formed when the acid loses a proton (${{\text{H}}^{+}}$). The stability of the conjugate base depends on the delocalization of the negative charge formed when the proton leaves the acid. When more number of electronegative atoms share the negative charge, the conjugate base becomes more stable.
Complete step by step answer:
There are two reasons by which carboxylic acid is a stronger acid than phenol. Let us see those reasons.
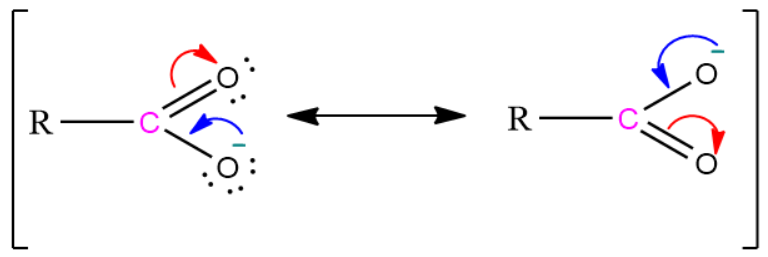
1) The conjugate base of a carboxylic acid is a carboxylate ion. It is stabilized by two equivalent resonating structures in which the negative charge is delocalized among two electronegative oxygen atoms.
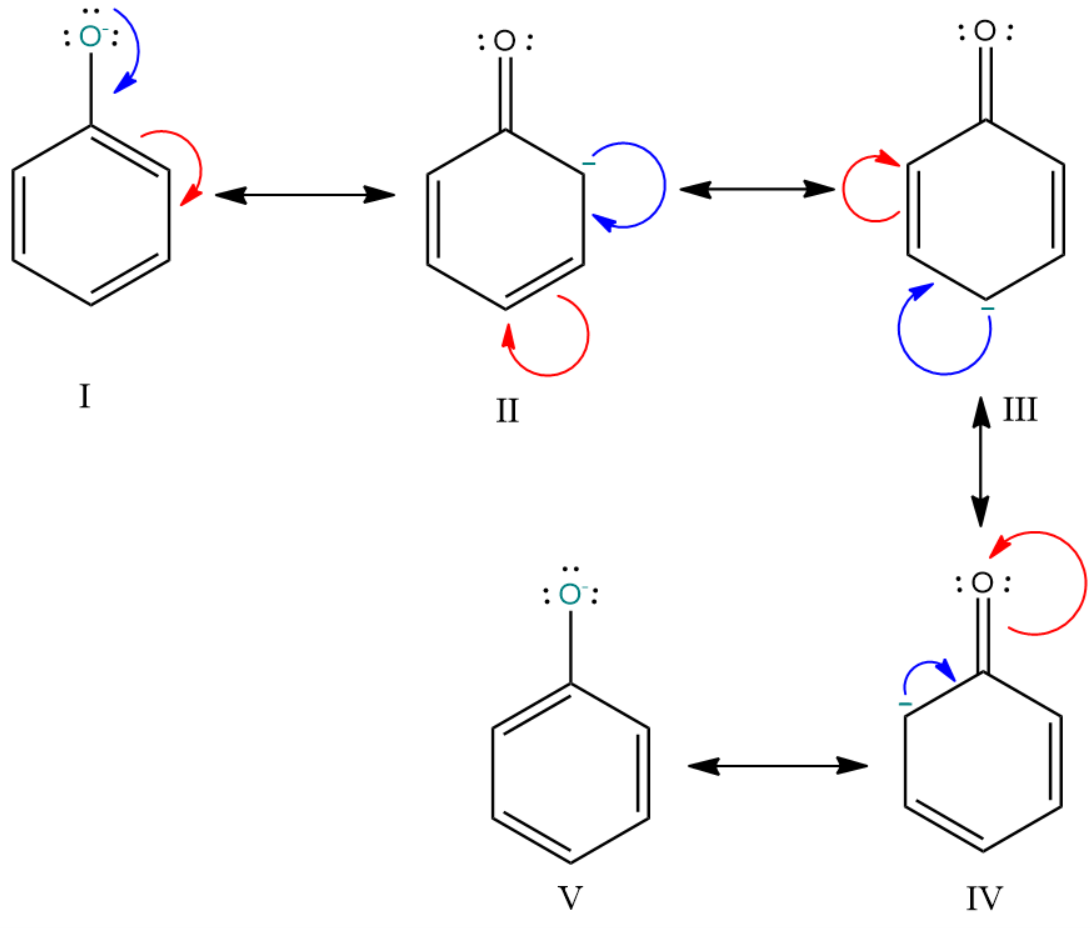
On the other hand, the conjugate base of phenol is a phenoxide ion and it has five resonance structures in which three of the structures have the negative charge at the less electronegative carbon atoms.
As we know that the stability of the conjugate base increases when the negative charge is delocalised among the electronegative atoms. Hence the carboxylate ion i.e. the conjugate base of the carboxylic acid is more stable than the phenoxide ion i.e. the conjugate base of phenol.
Resonating structure of phenoxide ion:
Resonating structure of carboxylate ion:
2) Secondly, the negative charge is delocalised over two negative oxygen atoms in carboxylate ion but in case of phenoxide ion, it is less effectively delocalised over one oxygen atom three less electronegative carbon atoms. Thus, the carboxylate ion is more stable than phenoxide ion, so carboxylic acid is more acidic than phenol.
Note:
The substituents which are present in the carbon chain or the ring may affect the stability of the conjugate base and thus, also affect the acidity of the acids. If there is an electron-withdrawing group it can stabilize the conjugate base by withdrawing the negative towards itself making. But if there is an electron releasing group, then it can destabilize the conjugate base by releasing electrons. Thus acidity increases in the presence of electron-withdrawing groups while it decreases in the presence of electron-releasing groups.
The acidic strength depends on the stability of the conjugate base which is formed when the acid loses a proton (${{\text{H}}^{+}}$). The stability of the conjugate base depends on the delocalization of the negative charge formed when the proton leaves the acid. When more number of electronegative atoms share the negative charge, the conjugate base becomes more stable.
Complete step by step answer:
There are two reasons by which carboxylic acid is a stronger acid than phenol. Let us see those reasons.
1) The conjugate base of a carboxylic acid is a carboxylate ion. It is stabilized by two equivalent resonating structures in which the negative charge is delocalized among two electronegative oxygen atoms.
On the other hand, the conjugate base of phenol is a phenoxide ion and it has five resonance structures in which three of the structures have the negative charge at the less electronegative carbon atoms.
As we know that the stability of the conjugate base increases when the negative charge is delocalised among the electronegative atoms. Hence the carboxylate ion i.e. the conjugate base of the carboxylic acid is more stable than the phenoxide ion i.e. the conjugate base of phenol.
Resonating structure of phenoxide ion:

Resonating structure of carboxylate ion:

2) Secondly, the negative charge is delocalised over two negative oxygen atoms in carboxylate ion but in case of phenoxide ion, it is less effectively delocalised over one oxygen atom three less electronegative carbon atoms. Thus, the carboxylate ion is more stable than phenoxide ion, so carboxylic acid is more acidic than phenol.
Note:
The substituents which are present in the carbon chain or the ring may affect the stability of the conjugate base and thus, also affect the acidity of the acids. If there is an electron-withdrawing group it can stabilize the conjugate base by withdrawing the negative towards itself making. But if there is an electron releasing group, then it can destabilize the conjugate base by releasing electrons. Thus acidity increases in the presence of electron-withdrawing groups while it decreases in the presence of electron-releasing groups.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




