
$AgN{O_3}\,\left( {aq} \right)$ was added to an aqueous $KCl$ solution gradually and the conductivity of the solution was measured. The plot of conductance ($\Lambda $) versus the volume of $AgN{O_3}$ is
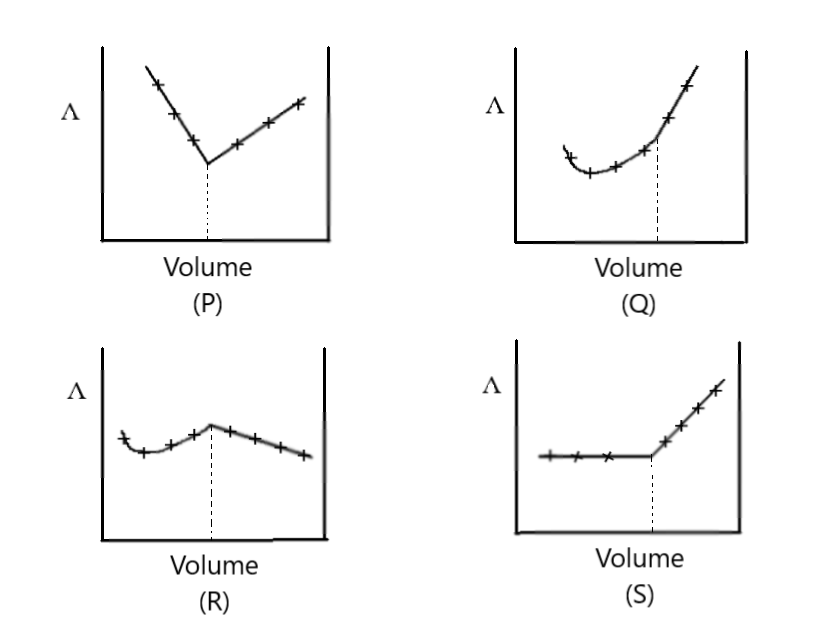
A) P
B) Q
C) R
D) S

Answer
573.9k+ views
Hint: The conductivity of a solution depends upon the quantity of ions at the same time on the mobility of ions present in the solution
Usually $C{l^ - }$ and $N{O_3}^ - $ have the same mobility hence both will have the similar effect on the value of conductance
Complete step by step solution:
The reaction for this question will be
$AgN{O_3} + KCl \to KN{O_3} + AgCl \downarrow $
Initially the solution consist only of two type of ions from $KCl$ that are ${K^ + }$ and $C{l^ - }$
Now when $AgN{O_3}$ is added to this solution there will be four types of ions in the solution as follow
${K^ + },C{l^ - },A{g^ + }and\,N{O_3}^ - $
As we know that $A{g^ + }$ will react with $C{l^ - }$ to form a white precipitate of $AgCl$
Now this will reduce the amount of $C{l^ - }$ but this decrease ions will be compensated by the $N{O_3}^ - $ ions which have the same mobility as $C{l^ - }$
Now whatever the change happened in conductance value due to elimination of $C{l^ - }$ ions was compensated by $N{O_3}^ - $ ions hence the value of conductance remains constant for some time as respect to volume of $AgN{O_3}$
After some time all the $C{l^ - }$ ions will be consumed by $A{g^ + }$ for forming white precipitate of $AgCl$
And further addition of $AgN{O_3}$will increase amount of $A{g^ + }$ causing a rapid increase in the conductance value of solution
The reaction for this question will be
$AgN{O_3} + KCl \to KN{O_3} + AgCl \downarrow $
Hence, we find out that the conductivity of the solution initially remain constant but after excess addition of $AgN{O_3}$it increased rapidly
Thus, the option is “D” the correct solution for the given question.
Note:
Further the conductivity of a solution also depends upon the size of the ion, on the dielectric constant and viscosity of solvent and the temperature of the solution
The $S.I$ unit for measurement of conductance is Siemens $\left( S \right)$
Usually $C{l^ - }$ and $N{O_3}^ - $ have the same mobility hence both will have the similar effect on the value of conductance
Complete step by step solution:
The reaction for this question will be
$AgN{O_3} + KCl \to KN{O_3} + AgCl \downarrow $
Initially the solution consist only of two type of ions from $KCl$ that are ${K^ + }$ and $C{l^ - }$
Now when $AgN{O_3}$ is added to this solution there will be four types of ions in the solution as follow
${K^ + },C{l^ - },A{g^ + }and\,N{O_3}^ - $
As we know that $A{g^ + }$ will react with $C{l^ - }$ to form a white precipitate of $AgCl$
Now this will reduce the amount of $C{l^ - }$ but this decrease ions will be compensated by the $N{O_3}^ - $ ions which have the same mobility as $C{l^ - }$
Now whatever the change happened in conductance value due to elimination of $C{l^ - }$ ions was compensated by $N{O_3}^ - $ ions hence the value of conductance remains constant for some time as respect to volume of $AgN{O_3}$
After some time all the $C{l^ - }$ ions will be consumed by $A{g^ + }$ for forming white precipitate of $AgCl$
And further addition of $AgN{O_3}$will increase amount of $A{g^ + }$ causing a rapid increase in the conductance value of solution
The reaction for this question will be
$AgN{O_3} + KCl \to KN{O_3} + AgCl \downarrow $
Hence, we find out that the conductivity of the solution initially remain constant but after excess addition of $AgN{O_3}$it increased rapidly
Thus, the option is “D” the correct solution for the given question.
Note:
Further the conductivity of a solution also depends upon the size of the ion, on the dielectric constant and viscosity of solvent and the temperature of the solution
The $S.I$ unit for measurement of conductance is Siemens $\left( S \right)$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




