
When $AgN{O_3}$ is added to a solution of $Co{\left( {N{H_3}} \right)_5}C{l_3}$, the precipitate of $AgCl$ shows two ionized chloride ions. This means:
A. Only two chlorine atoms satisfy primary valency and one secondary valency
B. One chlorine atom satisfies primary as well as secondary valency
C. Two chlorine atoms satisfy primary valency
D. Three chlorine atoms satisfy secondary valency
Answer
571.8k+ views
Hint: Primary Valency: - These are the valencies which the metals show in the formation of its simple salts. For example in the formation of $CuS{O_4}$ ,the primary valency of Cu is 2.
Secondary Valency: - These are the valencies which the metal atom or cation shows towards ligands (neutral molecules or ions) in the formation of its complexions. The secondary valencies of a metal atom or ion are equal to the number of coordinate bonds which the ligands form with it. It is also called the coordination number of the cation. For example, in ${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$ , the secondary valencies of copper are equal to 4.
Complete step by step answer:
Let us calculate the primary and secondary valency of the complex \[\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}\]
As we know that primary valency is the charge on the metal atom.
\[\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}\]
\[ \Rightarrow x + 5 \times 0 + ( - 1) - 2 = 0\]
\[ \Rightarrow x - 3 = 0\]
\[ \Rightarrow x = + 3\]
So we can see that the charge on the metal atom, i.e. Cobalt is \[ + 3\] which means that the primary valency is 3.
Now we will calculate the secondary valency.
\Secondary valency \[ = \] Number of lone pairs of electrons donated to the metal atom by the ligands.
Here we can see five ammonia will donate five lone pairs and one chlorine will donate one lone pair which is equal to 6. Therefore, the secondary valency will be 6.
The structure is:
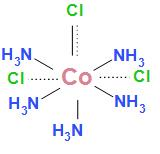
The dotted lines represent primary valency and the wedge line represents secondary valency.
As we can see in the above diagram that one chlorine atom satisfies both primary as well as secondary valency. The other two chlorine atoms satisfy primary valency.
Therefore we can conclude that one chlorine atom satisfies primary as well as secondary valency and two chlorine atoms satisfy primary valency.
So, the correct answer is Option B,C.
Note: The concept of primary and secondary valency comes from the postulates of Werner’s theory.
The important postulates of Werner’s theory are:
1.Metal possesses two types of valencies called (a) Primary Valency (b) Secondary Valency.
2.Every metal atom tends to satisfy both its primary and secondary valencies. Primary valencies are satisfied with negative ions while secondary valencies are satisfied by ligands i.e. neutral molecules or negative ions.
3.Primary Valencies are non-directional.
4.The ligands satisfying secondary valencies are always directed towards fixed positions in space and thus give definite geometry to the complex. Four such bonds may give square planar or tetrahedral geometry to the complex whereas six such valencies may give octahedral geometry to it. Two secondary valencies are arranged linearly.
5.Every metal atom has a fixed number of secondary valencies or co-ordination numbers.
Secondary Valency: - These are the valencies which the metal atom or cation shows towards ligands (neutral molecules or ions) in the formation of its complexions. The secondary valencies of a metal atom or ion are equal to the number of coordinate bonds which the ligands form with it. It is also called the coordination number of the cation. For example, in ${\left[ {Cu{{\left( {N{H_3}} \right)}_4}} \right]^{2 + }}$ , the secondary valencies of copper are equal to 4.
Complete step by step answer:
Let us calculate the primary and secondary valency of the complex \[\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}\]
As we know that primary valency is the charge on the metal atom.
\[\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]C{l_2}\]
\[ \Rightarrow x + 5 \times 0 + ( - 1) - 2 = 0\]
\[ \Rightarrow x - 3 = 0\]
\[ \Rightarrow x = + 3\]
So we can see that the charge on the metal atom, i.e. Cobalt is \[ + 3\] which means that the primary valency is 3.
Now we will calculate the secondary valency.
\Secondary valency \[ = \] Number of lone pairs of electrons donated to the metal atom by the ligands.
Here we can see five ammonia will donate five lone pairs and one chlorine will donate one lone pair which is equal to 6. Therefore, the secondary valency will be 6.
The structure is:

The dotted lines represent primary valency and the wedge line represents secondary valency.
As we can see in the above diagram that one chlorine atom satisfies both primary as well as secondary valency. The other two chlorine atoms satisfy primary valency.
Therefore we can conclude that one chlorine atom satisfies primary as well as secondary valency and two chlorine atoms satisfy primary valency.
So, the correct answer is Option B,C.
Note: The concept of primary and secondary valency comes from the postulates of Werner’s theory.
The important postulates of Werner’s theory are:
1.Metal possesses two types of valencies called (a) Primary Valency (b) Secondary Valency.
2.Every metal atom tends to satisfy both its primary and secondary valencies. Primary valencies are satisfied with negative ions while secondary valencies are satisfied by ligands i.e. neutral molecules or negative ions.
3.Primary Valencies are non-directional.
4.The ligands satisfying secondary valencies are always directed towards fixed positions in space and thus give definite geometry to the complex. Four such bonds may give square planar or tetrahedral geometry to the complex whereas six such valencies may give octahedral geometry to it. Two secondary valencies are arranged linearly.
5.Every metal atom has a fixed number of secondary valencies or co-ordination numbers.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




