
(a)Distinguish between primary, secondary and tertiary amines.
(b)Explain the structure of fructose.
Answer
591.3k+ views
Hint:
(a) Try drawing the skeleton structure of primary, secondary and tertiary amines and then try to see the difference in the bonding of Carbon atoms with the Nitrogen.
(b) An attempt to explain the structure of fructose can be by drawing the Fischer Projection of glucose and convert the aldehyde group to a keto group by rearrangement.
Complete step by step answer:
(a) Amines are classified as primary, secondary, or tertiary by the number of carbons bonded to the nitrogen atom. Primary amine has one carbon bonded to the nitrogen. Secondary amine has two carbons bonded to the nitrogen, and tertiary amine has three carbons bonded to the nitrogen.
Primary Amine:
\[{{R}_{{}}}-N{{H}_{2}}\]
Secondary Amine:
\[{{R}_{2}}-NH\]
Tertiary Amine:
\[{{R}_{\begin{smallmatrix}
3 \\
\end{smallmatrix}}}-N\]
Where, R is an alkyl group.
To distinguish between the three types of amines we will study the distinguishing tests in the table given below for a better understanding,
In Addition to the above tests there is one more test which is considered as the main test in distinguishing between the three types of amine and is called the Nitrous Acid test
Preparation of Nitrous Acid ($HN{{O}_{2}}$):
$NaN{{O}_{2}}+HCl\xrightarrow[dil.]{cold}NaCl+HN{{O}_{2}}$
Primary Amine: All primary amines, except methyl amine react with nitrous acid in cold condition to give alcohol and liberate nitrogen gas.
${{C}_{2}}{{H}_{5}}-N{{H}_{2}}+HO-N=O\xrightarrow[cold]{NaN{{O}_{2}}+dil.HCl}{{C}_{2}}{{H}_{5}}-OH+{{N}_{2}}+{{H}_{2}}O$
Secondary Amine: Secondary Amines on reaction with nitrous acid forms N-nitroso amine which is a pale-yellow oil.
${{({{C}_{2}}{{H}_{5}})}_{2}}-N{{H}_{{}}}+HO-N=O\xrightarrow[cold]{NaN{{O}_{2}}+dil.HCl}{{({{C}_{2}}{{H}_{5}})}_{2}}N-N=O+{{H}_{2}}O$
Tertiary Amine: We can say that no reaction occurs because there is no distinguishing in the state or color of reagent as they do not form a new compound.
The above tests that we discussed are used to distinguish between primary, secondary and tertiary amines.
(b)We will try to form a structure of Fructose from the results of the tests that we will discuss below.
-Elemental analysis show that the molecular formula of fructose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$
-Reaction with HI: Complete reduction of fructose with concentrated HI and red phosphorus gives n-Hexane as the product.
$Fructose\xrightarrow[{}]{HI/P}C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$
-Fructose when dissolved in water gives a neutral solution. This indicates that fructose does not contain a carboxyl group like that of Glucose which has the same molecular formula.
-It reacts with acetic anhydride in the presence of pyridine as a catalyst to form Penta acetate. This shows that fructose has 5 hydroxyl groups.
-Fructose reacts with 1 mole of HCN only, this shows that there is either an aldehyde group or a ketone. Since we found out that the aldehyde group is absent, we conclude that a ketone group is present.
-Fructose is not oxidized by bromine water which shows the absence of the aldehyde group.
From the above tests we can conclude that fructose is a pentahydroxy hexanone. We will now try to draw the structure of fructose with the help of above tests.
Structure of Fructose:
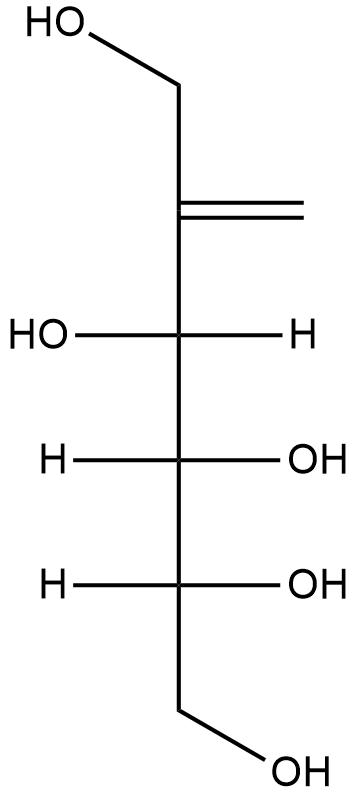
Hence the above structure obeys to the test findings and also the molecular and we can conclude that the correct structure of fructose is drawn above.
Note: At times students tend to confuse between isocyanate and isothiocyanate. The difference is that isothiocyanate has a Sulfur(S) atom and is a place of Oxygen(O) and hence we add the word 'thio'.
Students conclude the structure of fructose identical to glucose due to the same molecular weight and similarity in results of few tests. However, we must take the result of all tests in consideration before concluding the structure.
(a) Try drawing the skeleton structure of primary, secondary and tertiary amines and then try to see the difference in the bonding of Carbon atoms with the Nitrogen.
(b) An attempt to explain the structure of fructose can be by drawing the Fischer Projection of glucose and convert the aldehyde group to a keto group by rearrangement.
Complete step by step answer:
(a) Amines are classified as primary, secondary, or tertiary by the number of carbons bonded to the nitrogen atom. Primary amine has one carbon bonded to the nitrogen. Secondary amine has two carbons bonded to the nitrogen, and tertiary amine has three carbons bonded to the nitrogen.
Primary Amine:
\[{{R}_{{}}}-N{{H}_{2}}\]
Secondary Amine:
\[{{R}_{2}}-NH\]
Tertiary Amine:
\[{{R}_{\begin{smallmatrix}
3 \\
\end{smallmatrix}}}-N\]
Where, R is an alkyl group.
To distinguish between the three types of amines we will study the distinguishing tests in the table given below for a better understanding,
| Tests | Primary Amine | Secondary Amine | Tertiary Amine |
| Reaction with Hinsberg Reagent (Benzene sulphonyl chloride) and an alkali | Water Soluble compound formed | Water Insoluble compound formed | No reaction |
| Reaction of Amine with chloroform and KOH | Primary isocyanate formed with pungent odour | No reaction | No reaction |
| Reaction of Amine with $C{{S}_{2}}$ and HgCl | Primary isothiocyanate formed (Mustard Oil) | No reaction | No reaction |
In Addition to the above tests there is one more test which is considered as the main test in distinguishing between the three types of amine and is called the Nitrous Acid test
Preparation of Nitrous Acid ($HN{{O}_{2}}$):
$NaN{{O}_{2}}+HCl\xrightarrow[dil.]{cold}NaCl+HN{{O}_{2}}$
Primary Amine: All primary amines, except methyl amine react with nitrous acid in cold condition to give alcohol and liberate nitrogen gas.
${{C}_{2}}{{H}_{5}}-N{{H}_{2}}+HO-N=O\xrightarrow[cold]{NaN{{O}_{2}}+dil.HCl}{{C}_{2}}{{H}_{5}}-OH+{{N}_{2}}+{{H}_{2}}O$
Secondary Amine: Secondary Amines on reaction with nitrous acid forms N-nitroso amine which is a pale-yellow oil.
${{({{C}_{2}}{{H}_{5}})}_{2}}-N{{H}_{{}}}+HO-N=O\xrightarrow[cold]{NaN{{O}_{2}}+dil.HCl}{{({{C}_{2}}{{H}_{5}})}_{2}}N-N=O+{{H}_{2}}O$
Tertiary Amine: We can say that no reaction occurs because there is no distinguishing in the state or color of reagent as they do not form a new compound.
The above tests that we discussed are used to distinguish between primary, secondary and tertiary amines.
(b)We will try to form a structure of Fructose from the results of the tests that we will discuss below.
-Elemental analysis show that the molecular formula of fructose is ${{C}_{6}}{{H}_{12}}{{O}_{6}}$
-Reaction with HI: Complete reduction of fructose with concentrated HI and red phosphorus gives n-Hexane as the product.
$Fructose\xrightarrow[{}]{HI/P}C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}$
-Fructose when dissolved in water gives a neutral solution. This indicates that fructose does not contain a carboxyl group like that of Glucose which has the same molecular formula.
-It reacts with acetic anhydride in the presence of pyridine as a catalyst to form Penta acetate. This shows that fructose has 5 hydroxyl groups.
-Fructose reacts with 1 mole of HCN only, this shows that there is either an aldehyde group or a ketone. Since we found out that the aldehyde group is absent, we conclude that a ketone group is present.
-Fructose is not oxidized by bromine water which shows the absence of the aldehyde group.
From the above tests we can conclude that fructose is a pentahydroxy hexanone. We will now try to draw the structure of fructose with the help of above tests.
Structure of Fructose:

Hence the above structure obeys to the test findings and also the molecular and we can conclude that the correct structure of fructose is drawn above.
Note: At times students tend to confuse between isocyanate and isothiocyanate. The difference is that isothiocyanate has a Sulfur(S) atom and is a place of Oxygen(O) and hence we add the word 'thio'.
Students conclude the structure of fructose identical to glucose due to the same molecular weight and similarity in results of few tests. However, we must take the result of all tests in consideration before concluding the structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




