
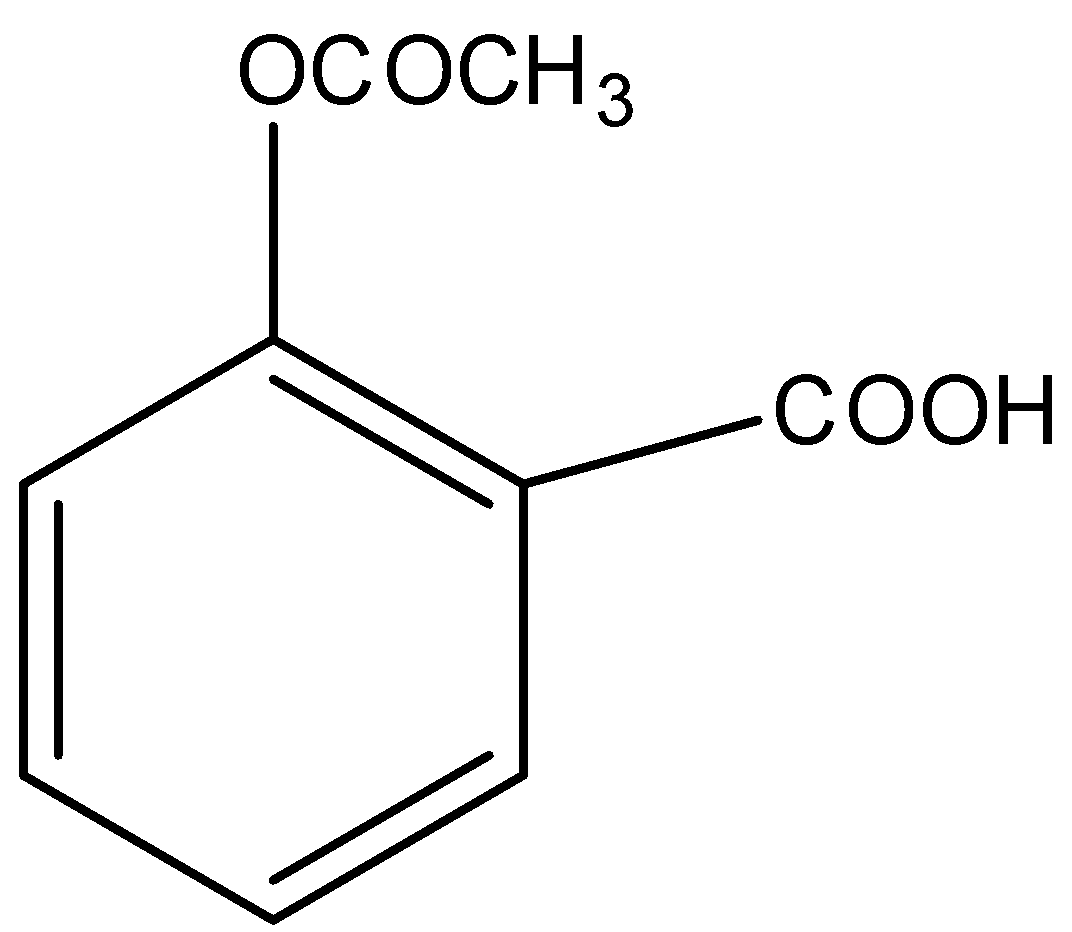
Acetyl salicylic acid \[(mol.\;wt.\; = 180)\] called aspirin is a pain killer with \[pKa\, = 6\]. It two tablets each of \[0.09\;gm\] mass containing aspirin are dissolved in \[100\;mL\;\] solution. Its $pH$ will be:
A.$0.5$
B.$1.0$
C.$4.0$
D.$2.0$

Answer
578.1k+ views
Hint:$pH$ is a scale used to determine the acidity or basicity of an aqueous solution. It indicates the concentration of ${H^ + }$ or $O{H^ - }$ ions in the solution. In acidic and basic solutions, the concentration of hydrogen ions and hydroxide ions depends on the molarity of the solution. It is given as \[pH = - log{{ }}\left[ \text{concentration of H^+} \right]\]
The formula used: $pH\, = \dfrac{1}{2}\left[ {pKa - \log C} \right]$ where, $pKa$ is the negative log of the acid dissociation constant. The lower the value of $pKa$ the more acid will dissociate into ions in water.
So, to solve this we need to calculate the number of moles (concentration) and then calculate the \[pH\] using the given $pKa$ value and concentration of acetylsalicylic acid.
Complete step by step answer:
Given, \[pKa\, = 6\] and \[mass = 0.09\;gm\]
-Now we will calculate the total no. of moles in two tablets of Acetylsalicylic acid,
$No.\,of\,moles = \dfrac{{given\,mass}}{{molar\,mass}}$
$No.\,of\,moles = \dfrac{{2 \times 0.09}}{{180}} = 0.001$
$M = \dfrac{{no.\,of\,moles}}{{volume\,(\,l)}} = \dfrac{{0.001 \times 1000}}{{100}} = 0.01$$(where\,M = molarity)$
-Now, the $pH$ will be; $pH\, = \dfrac{1}{2}\left[ {pKa - \log C} \right]$
$pH\, = \dfrac{1}{2}\left[ {6 - \log 0.01} \right] = \dfrac{1}{2}\left[ {6 - \left( { - 2} \right)} \right] = 4$
-Hence, the $pH$ of the Acetyl salicylic acid will be $4.0$
Therefore, Option (C) is correct.
Note:
Acetylsalicylic acid is a monoprotic acid. Monoprotic acids are weak acids which means that they can only donate one lone pair of electrons. This $pKa$ is defined as the value at which a chemical species accepts or donates a proton. The lower the $pKa$ value, the stronger will be the acid and greater will be its strength to donate a proton in aqueous solution. Acetylsalicylic acid has a variety of uses like relieving pain and swelling, conditions like flu, headache etcetera, and reducing the risk of cardiovascular diseases in people that have a high risk. There are some particular inflammatory conditions in which aspirin is used for treatment as Kawasaki disease, pericarditis, and rheumatic fever. The only thing that needs to be taken care of is its dose while using aspirin.
The formula used: $pH\, = \dfrac{1}{2}\left[ {pKa - \log C} \right]$ where, $pKa$ is the negative log of the acid dissociation constant. The lower the value of $pKa$ the more acid will dissociate into ions in water.
So, to solve this we need to calculate the number of moles (concentration) and then calculate the \[pH\] using the given $pKa$ value and concentration of acetylsalicylic acid.
Complete step by step answer:
Given, \[pKa\, = 6\] and \[mass = 0.09\;gm\]
-Now we will calculate the total no. of moles in two tablets of Acetylsalicylic acid,
$No.\,of\,moles = \dfrac{{given\,mass}}{{molar\,mass}}$
$No.\,of\,moles = \dfrac{{2 \times 0.09}}{{180}} = 0.001$
$M = \dfrac{{no.\,of\,moles}}{{volume\,(\,l)}} = \dfrac{{0.001 \times 1000}}{{100}} = 0.01$$(where\,M = molarity)$
-Now, the $pH$ will be; $pH\, = \dfrac{1}{2}\left[ {pKa - \log C} \right]$
$pH\, = \dfrac{1}{2}\left[ {6 - \log 0.01} \right] = \dfrac{1}{2}\left[ {6 - \left( { - 2} \right)} \right] = 4$
-Hence, the $pH$ of the Acetyl salicylic acid will be $4.0$
Therefore, Option (C) is correct.
Note:
Acetylsalicylic acid is a monoprotic acid. Monoprotic acids are weak acids which means that they can only donate one lone pair of electrons. This $pKa$ is defined as the value at which a chemical species accepts or donates a proton. The lower the $pKa$ value, the stronger will be the acid and greater will be its strength to donate a proton in aqueous solution. Acetylsalicylic acid has a variety of uses like relieving pain and swelling, conditions like flu, headache etcetera, and reducing the risk of cardiovascular diseases in people that have a high risk. There are some particular inflammatory conditions in which aspirin is used for treatment as Kawasaki disease, pericarditis, and rheumatic fever. The only thing that needs to be taken care of is its dose while using aspirin.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




