
According to the de-Broglie explanation of Bohr's second postulate of quantization, the standing particle wave on a circular orbit for $n=4$ is given by
$\begin{align}
& A.2\pi {{r}_{n}}=\dfrac{4}{\lambda } \\
& B.\dfrac{2\pi }{\lambda }=4{{r}_{n}} \\
& C.2\pi {{r}_{n}}=4\lambda \\
& D.\dfrac{\lambda }{2\pi }=4{{r}_{n}} \\
\end{align}$
Answer
590.4k+ views
Hint: In accordance to de Broglie’s explanation of the second postulate of Bohr, there an assumption is created that the integral number of wavelengths should be equivalent to the circumference of circular orbit. This integral multiple is found to be similar to the quantization number. This may help you to solve this question.
Complete answer:
first of all let us check what the second postulate of Bohr is.
Bohr described the stable orbits in his second postulate. According to this postulate, an electron is found to be revolving around the nucleus of an atom. In this revolution, the angular momentum is calculated as the integral multiple of $\dfrac{h}{2\pi }$ . Where $h$ be the Planck’s constant. Therefore the angular momentum abbreviated as $L$ of the orbiting electron is calculated by the expression,
$L=\dfrac{nh}{2\pi }$
Where $n$ be the principal quantum number of the atom.
Here in this question, it is given that,
$n=4$
The postulate tells that the integral number of wavelengths will be fitted in the circumference of a circular orbit. The quantisation number comes out to be the equal to the integral multiple.
Therefore we can write that,
$\begin{align}
& 2\pi {{r}_{n}}=n\lambda \\
& \because n=4 \\
& 2\pi {{r}_{n}}=4\lambda \\
\end{align}$
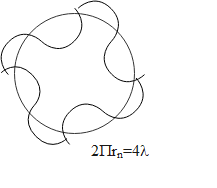
So, the correct answer is “Option C”.
Note:
Quantization of angular momentum can be defined as the radius of the orbit is found to be discrete which means it is quantised. Not only the radius, but also the energy is also quantised. Bohr proposed that the discrete lines visible in the spectrum of the hydrogen atom are formed due to the transitions of an electron occurring from one permitted orbit or energy level to another.
Complete answer:
first of all let us check what the second postulate of Bohr is.
Bohr described the stable orbits in his second postulate. According to this postulate, an electron is found to be revolving around the nucleus of an atom. In this revolution, the angular momentum is calculated as the integral multiple of $\dfrac{h}{2\pi }$ . Where $h$ be the Planck’s constant. Therefore the angular momentum abbreviated as $L$ of the orbiting electron is calculated by the expression,
$L=\dfrac{nh}{2\pi }$
Where $n$ be the principal quantum number of the atom.
Here in this question, it is given that,
$n=4$
The postulate tells that the integral number of wavelengths will be fitted in the circumference of a circular orbit. The quantisation number comes out to be the equal to the integral multiple.
Therefore we can write that,
$\begin{align}
& 2\pi {{r}_{n}}=n\lambda \\
& \because n=4 \\
& 2\pi {{r}_{n}}=4\lambda \\
\end{align}$
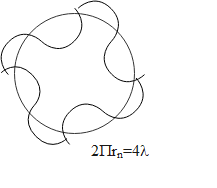
So, the correct answer is “Option C”.
Note:
Quantization of angular momentum can be defined as the radius of the orbit is found to be discrete which means it is quantised. Not only the radius, but also the energy is also quantised. Bohr proposed that the discrete lines visible in the spectrum of the hydrogen atom are formed due to the transitions of an electron occurring from one permitted orbit or energy level to another.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




