
a) Write the name and molecular formula of the first member of the homologous series of alkynes.
b) Write the next homologue of each of the following
i) \[{C_2}{H_4}\] ii) \[{C_4}{H_6}\].
Answer
573.3k+ views
Hint: Alkyne is a class of organic compound with molecular formula \[{C_n}{H_{2n - 2}}\]. Alkene is a class of compound with molecular formula \[{C_n}{H_{2n}}\]. The term homologue refers to the compound having one carbon more or less than the compound considered.
Complete step by step answer:
a) The first member of alkyne is acetylene represented as \[{C_2}{H_2}\]. It is also known as ethyne. The carbon-carbon bond present in ethyne is a triple bond and each carbon atom is attached to one hydrogen atom.
The structure of ethyne is:
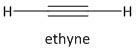
The carbon atom in ethyne is sp hybridized and the angle between the \[C \equiv C - H\] bonds is \[180^\circ C\] . The acetylene has \[s\] and \[p\]character in the ratio 1:1. The protons attached to carbon are acidic in nature and are taken up or abstracted with a base.
b)
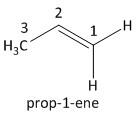
i) \[{C_2}{H_4}\]: This compound belongs to an alkene class of organic compound. It follows the general formula of alkene as \[{C_n}{H_{2n}}\], where \[n = 2\]. This is the simplest alkene known as ethene.
The next homologue of ethene has one carbon atom more than ethene i.e. propene. The value of \[n\] is \[3\]. The molecular formula is \[{C_3}{H_6}\] . The numbering of the carbon atom starts with the terminal carbon having the double bond.
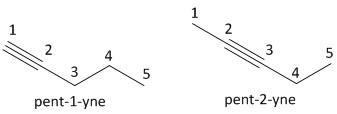
ii) \[{C_4}{H_6}\]: This compound belongs to an alkyne class of organic compound. It follows the general formula of alkyne as \[{C_n}{H_{2n - 2}}\], where \[n = 4\]. The compound is butyne having a triple bond.
The next homologue of butyne is pentyne having the number of carbon atoms equal to five. The value of \[n\] is \[5\]and the molecular formula is \[{C_5}{H_8}\] . The numbering of carbon atoms starts with the carbon atom in which the triple bonded carbon has a minimum number.
So, the correct answer is Option A.
Note: Alkane is referred as saturated hydrocarbon while alkene and alkyne are referred as unsaturated hydrocarbon. Alkynes are more closely attracted due to smaller size and greater force of attraction than alkene and alkane so it has higher boiling point.
Complete step by step answer:
a) The first member of alkyne is acetylene represented as \[{C_2}{H_2}\]. It is also known as ethyne. The carbon-carbon bond present in ethyne is a triple bond and each carbon atom is attached to one hydrogen atom.
The structure of ethyne is:

The carbon atom in ethyne is sp hybridized and the angle between the \[C \equiv C - H\] bonds is \[180^\circ C\] . The acetylene has \[s\] and \[p\]character in the ratio 1:1. The protons attached to carbon are acidic in nature and are taken up or abstracted with a base.
b)
i) \[{C_2}{H_4}\]: This compound belongs to an alkene class of organic compound. It follows the general formula of alkene as \[{C_n}{H_{2n}}\], where \[n = 2\]. This is the simplest alkene known as ethene.
The next homologue of ethene has one carbon atom more than ethene i.e. propene. The value of \[n\] is \[3\]. The molecular formula is \[{C_3}{H_6}\] . The numbering of the carbon atom starts with the terminal carbon having the double bond.

ii) \[{C_4}{H_6}\]: This compound belongs to an alkyne class of organic compound. It follows the general formula of alkyne as \[{C_n}{H_{2n - 2}}\], where \[n = 4\]. The compound is butyne having a triple bond.
The next homologue of butyne is pentyne having the number of carbon atoms equal to five. The value of \[n\] is \[5\]and the molecular formula is \[{C_5}{H_8}\] . The numbering of carbon atoms starts with the carbon atom in which the triple bonded carbon has a minimum number.

So, the correct answer is Option A.
Note: Alkane is referred as saturated hydrocarbon while alkene and alkyne are referred as unsaturated hydrocarbon. Alkynes are more closely attracted due to smaller size and greater force of attraction than alkene and alkane so it has higher boiling point.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




