
a) Write a chemical equation to prepare ammonia from ammonium chloride.
b) Write a chemical equation to identify $C{u^{2 + }}$ and $A{g^ + }$ ions with the application of ammonia?
c) Draw a labeled diagram showing flow charts for the manufacture of ammonia.
Answer
573.6k+ views
Hint:There are three well-known hydrides of nitrogen with hydrogen- ammonia, hydrazine and hydrazoic acid. Industrially, ammonia is prepared by Haber’s process using nitrogen and hydrogen gas. Ammonia is also present in traces in the atmosphere.
b)Ammonia is a colorless gas and has a pungent odor. It has a trigonal pyramidal structure. It also behaves as a lewis base. It is commercially important in the manufacture of many chemicals.
c)Ammonia is manufactured in the laboratory using hydrogen and nitrogen gas. The catalysts required for this process are iron oxide and a small amount of ${K_2}O,A{l_2}{O_3}$ . Industrially it is manufactured using Haber’s process.
Complete step by step answer:
a).Ammonia is formed in the atmosphere by the bacterial decomposition of nitrogenous matter of plants and animals. In laboratories it can be prepared by using different methods. Ammonia can be prepared from ammonium chloride in the following ways-
When ammonium chloride is heated with caustic soda(sodium hydroxide) , ammonia is formed.
$N{H_4}Cl + NaOH \to N{H_3} + NaCl + {H_2}O$
It can also be prepared using slaked lime(calcium hydroxide)-
$2N{H_4}Cl + Ca{(OH)_2} \to 2N{H_3} + CaC{l_2} + 2{H_2}O$
Ammonia is also formed when ammonium chloride is heated with litharge(lead oxide)
$2N{H_4}Cl + PbO \to 2N{H_3} + PbC{l_2} + {H_2}O$
b).Ammonia behaves as a lewis base. In ammonia, nitrogen has a lone pair of electrons that is available for donation. Ammonia can donate this lone pair thus behaving as a base and can form linkage or bonds with other metal ions. This property finds application in detection of metal ions.
It is also used to detect ions such as $C{u^{2 + }}$ and $A{g^ + }$ .
A solution containing copper ions that is blue colored when treated with ammonia forms a complex of ammonia and copper and the solution becomes deep blue in color. The reaction is-
$C{u^{2 + }}(aq) + 4N{H_3}(aq) \to \left[ {Cu{{(N{H_3})}_4}} \right]_{(aq)}^{2 + }$
Thus, copper ions can be detected.
A solution containing silver chloride has a white precipitate of silver chloride. When ammonia is passed through such a solution it becomes colorless. Ammonia forms a complex with silver ions.
$AgCl(s) + 2N{H_3}(aq) \to [Ag(N{H_3})]Cl(aq)$
c).The flow chart for the manufacture of ammonia can be drawn as-
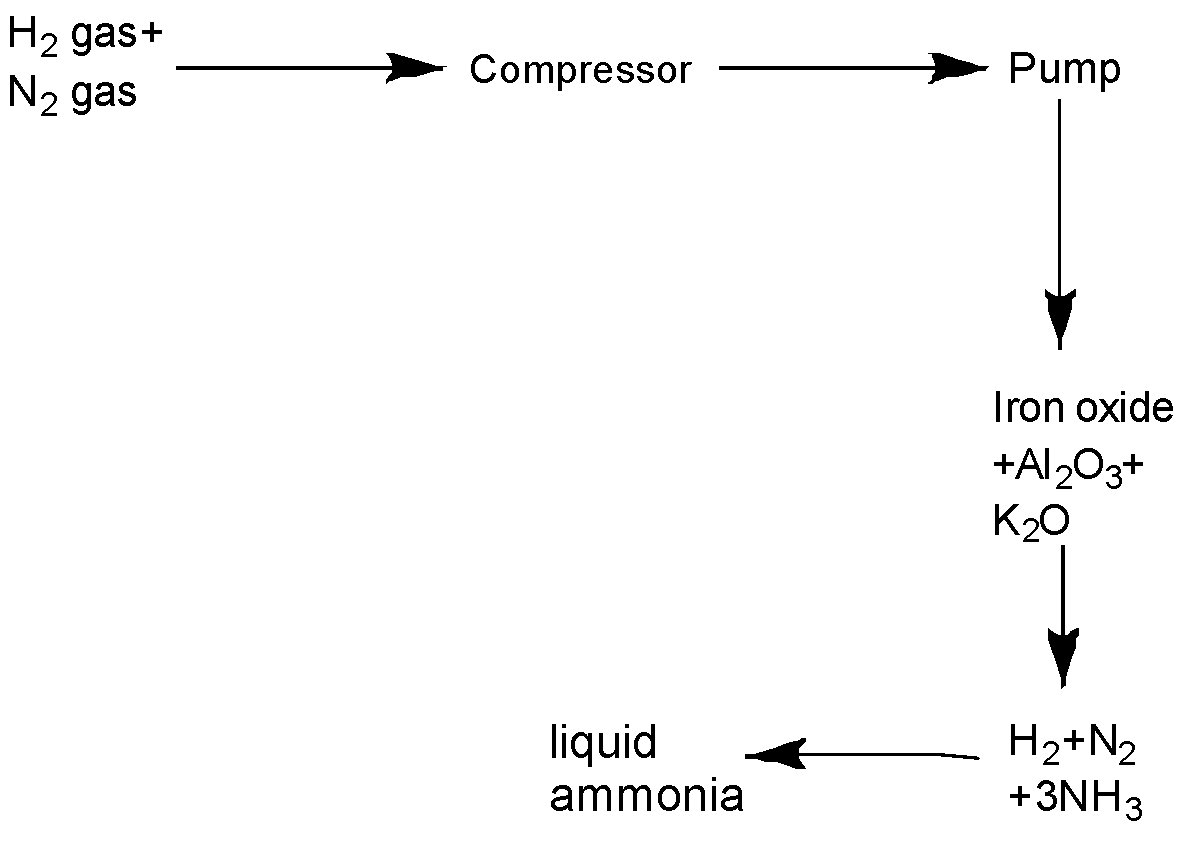
The reaction for the production of ammonia can be represented as-
$3{H_2}(g) + {N_2}(g) \to 2N{H_3}(g)$
The optimum conditions for the production of ammonia are a pressure of $200atm$ and a temperature of $700K$ . This process requires a catalyst like iron oxide mixed with small amounts of ${K_2}O$ and $A{l_2}{O_3}$ so that equilibrium is attained quickly.
Note:
a).Ammonia is a colorless gas with a characteristic pungent odor. It is highly soluble in water. Ammonia is neither combustible nor a supporter of combustion. It is a Lewis base. It is used in the manufacture of ammonia by Ostwald’s process.
b).Any compound having the ability to donate electrons is called a Lewis base. Ammonia gas is also used to precipitate the hydroxides of many metal ions. In inorganic qualitative analysis $A{g^ + }$ is categorized as Group $1$ radical and $C{u^{2 + }}$ as Group $2$ basic radical.
c).Industrially ammonia is manufactured using Haber’s process. Ammonia is used in the manufacture of many chemicals like nitric acid, sodium bicarbonate and ammonium compounds. It is also used in the manufacture of fertilizer like urea.
b)Ammonia is a colorless gas and has a pungent odor. It has a trigonal pyramidal structure. It also behaves as a lewis base. It is commercially important in the manufacture of many chemicals.
c)Ammonia is manufactured in the laboratory using hydrogen and nitrogen gas. The catalysts required for this process are iron oxide and a small amount of ${K_2}O,A{l_2}{O_3}$ . Industrially it is manufactured using Haber’s process.
Complete step by step answer:
a).Ammonia is formed in the atmosphere by the bacterial decomposition of nitrogenous matter of plants and animals. In laboratories it can be prepared by using different methods. Ammonia can be prepared from ammonium chloride in the following ways-
When ammonium chloride is heated with caustic soda(sodium hydroxide) , ammonia is formed.
$N{H_4}Cl + NaOH \to N{H_3} + NaCl + {H_2}O$
It can also be prepared using slaked lime(calcium hydroxide)-
$2N{H_4}Cl + Ca{(OH)_2} \to 2N{H_3} + CaC{l_2} + 2{H_2}O$
Ammonia is also formed when ammonium chloride is heated with litharge(lead oxide)
$2N{H_4}Cl + PbO \to 2N{H_3} + PbC{l_2} + {H_2}O$
b).Ammonia behaves as a lewis base. In ammonia, nitrogen has a lone pair of electrons that is available for donation. Ammonia can donate this lone pair thus behaving as a base and can form linkage or bonds with other metal ions. This property finds application in detection of metal ions.
It is also used to detect ions such as $C{u^{2 + }}$ and $A{g^ + }$ .
A solution containing copper ions that is blue colored when treated with ammonia forms a complex of ammonia and copper and the solution becomes deep blue in color. The reaction is-
$C{u^{2 + }}(aq) + 4N{H_3}(aq) \to \left[ {Cu{{(N{H_3})}_4}} \right]_{(aq)}^{2 + }$
Thus, copper ions can be detected.
A solution containing silver chloride has a white precipitate of silver chloride. When ammonia is passed through such a solution it becomes colorless. Ammonia forms a complex with silver ions.
$AgCl(s) + 2N{H_3}(aq) \to [Ag(N{H_3})]Cl(aq)$
c).The flow chart for the manufacture of ammonia can be drawn as-

The reaction for the production of ammonia can be represented as-
$3{H_2}(g) + {N_2}(g) \to 2N{H_3}(g)$
The optimum conditions for the production of ammonia are a pressure of $200atm$ and a temperature of $700K$ . This process requires a catalyst like iron oxide mixed with small amounts of ${K_2}O$ and $A{l_2}{O_3}$ so that equilibrium is attained quickly.
Note:
a).Ammonia is a colorless gas with a characteristic pungent odor. It is highly soluble in water. Ammonia is neither combustible nor a supporter of combustion. It is a Lewis base. It is used in the manufacture of ammonia by Ostwald’s process.
b).Any compound having the ability to donate electrons is called a Lewis base. Ammonia gas is also used to precipitate the hydroxides of many metal ions. In inorganic qualitative analysis $A{g^ + }$ is categorized as Group $1$ radical and $C{u^{2 + }}$ as Group $2$ basic radical.
c).Industrially ammonia is manufactured using Haber’s process. Ammonia is used in the manufacture of many chemicals like nitric acid, sodium bicarbonate and ammonium compounds. It is also used in the manufacture of fertilizer like urea.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




