
A solid AB has NaCl type structure with edge length 580.4pm . The radius of ${A^ + }$ is 100pm . What is the radius (in pm) of ${B^ - }$?
A.190.20
B.540.13
C.525.23
D.78.12
Answer
597.3k+ views
Hint: Sodium chloride is an ionic compound in which sodium and chloride ions are in the ratio of $1:1$.The crystal structure is an ordered array of atoms, ions, or molecules. In the case of NaCl structure, each atom has six nearest neighbors, with octahedral geometry. This arrangement is known as cubic close packing.
Formula used:
${r_c} + {r_a} = \dfrac{a}{2}$
Where, ${r_c} = $radius of cation, ${r_a} = $radius of anion and a$ = $edge length
Complete step by step answer:
Crystal structure is obtained by attaching atoms, groups of atoms or molecules. This structure occurs from the intrinsic nature of the constituent particles to produce symmetric patterns.
NaCl has a cubic unit cell. It is best thought of as a face centered cubic array of anions with an interpreting fcc cation lattice (or vice versa). Its structure is as shown:
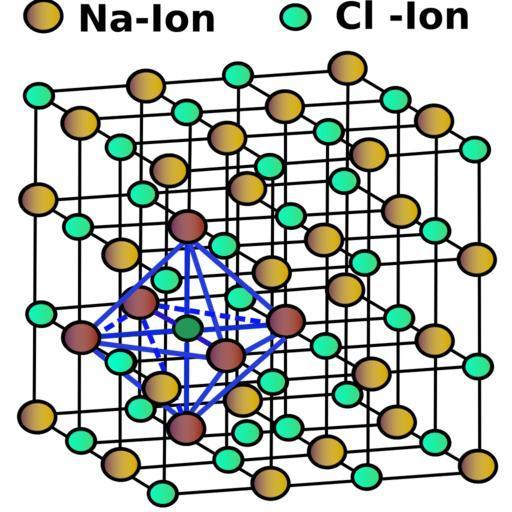
Now, in the given question, the edge length is 580.4pm and the radius of cation is 100pm. Let’s find out the radius of anion.
As we know that $NaCl$ has fcc structure, thus according to the formula,
${r_c} + {r_a} = \dfrac{a}{2}$
$100 + {r_a} = \dfrac{{580.4}}{2}$
$ = 290.2pm$
Now, $100 + {r_a} = 290.2pm$
Therefore, ${r_a} = 290.2 - 100$
$ = 190.2pm$
Hence, option A is correct.
Note:
In its aqueous state, NaCl acts as a good conductor of electricity due to the free movement of the ions. It is easily soluble in water and partially soluble in other liquids. It is a major raw material in the industrial manufacturing of various chemicals such as sodium carbonate, sodium hydrogen carbonate etc.
Formula used:
${r_c} + {r_a} = \dfrac{a}{2}$
Where, ${r_c} = $radius of cation, ${r_a} = $radius of anion and a$ = $edge length
Complete step by step answer:
Crystal structure is obtained by attaching atoms, groups of atoms or molecules. This structure occurs from the intrinsic nature of the constituent particles to produce symmetric patterns.
NaCl has a cubic unit cell. It is best thought of as a face centered cubic array of anions with an interpreting fcc cation lattice (or vice versa). Its structure is as shown:

Now, in the given question, the edge length is 580.4pm and the radius of cation is 100pm. Let’s find out the radius of anion.
As we know that $NaCl$ has fcc structure, thus according to the formula,
${r_c} + {r_a} = \dfrac{a}{2}$
$100 + {r_a} = \dfrac{{580.4}}{2}$
$ = 290.2pm$
Now, $100 + {r_a} = 290.2pm$
Therefore, ${r_a} = 290.2 - 100$
$ = 190.2pm$
Hence, option A is correct.
Note:
In its aqueous state, NaCl acts as a good conductor of electricity due to the free movement of the ions. It is easily soluble in water and partially soluble in other liquids. It is a major raw material in the industrial manufacturing of various chemicals such as sodium carbonate, sodium hydrogen carbonate etc.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




