
A new $\text{C}-\text{C}$ bond formation is possible in:
A. Cannizaro reaction
B. Rosenmunds reduction
C. Clemmensen reduction
D. Reimer-Tiemann reaction
Answer
590.1k+ views
Hint: New $\left( \text{C}-\text{C} \right)$ bond formation is taking place means carbon atom is forming bond with new carbon atom. This can be known only looking at the reaction in the methods given in the options. These all reactions include carbonyl compound either aldehyde $\left( -\text{CHO} \right)$ or ketone
Complete answer:
Let us discuss this question, by discussing the options and there particular reactions:
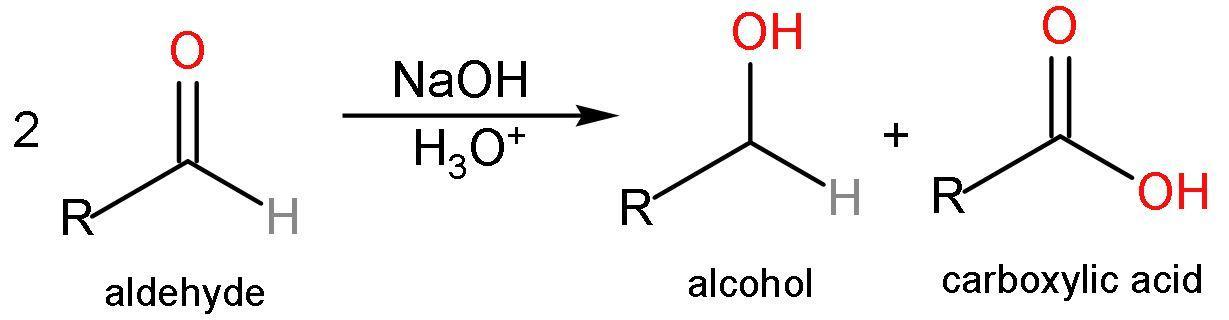
A. Cannizaro reaction: It is a chemical reaction which involves the disproportionation reaction of two molecules initiated by a base of a non-enolizable (ability to convert to $\text{R}-\text{OH}$) aldehyde to give a carboxylic acid and primary alcohol. No new $\left( \text{C}-\text{C} \right)$ bond formation is taking place. The reaction is
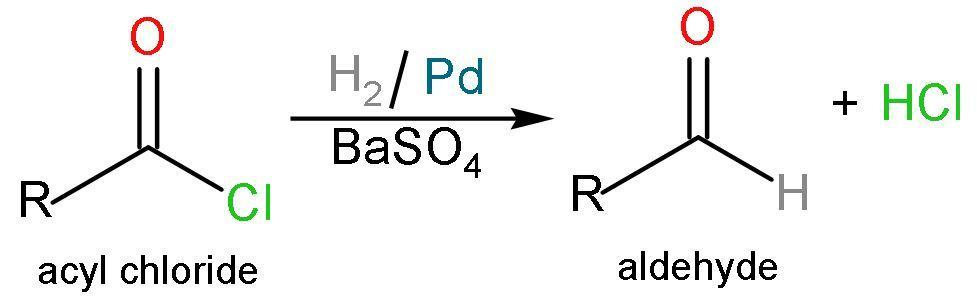
B. Rosenmunds reduction: Rosenmunds reduction reaction is a catalytic hydrogenation process where acyl chlorides are converted into aldehydes by passing hydrogen gas over palladium poisoned by barium sulphate. The barium sulphate reduces the activity of the palladium due to its low surface area, thereby preventing over reduction. No new $\left( \text{C}-\text{C} \right)$ bond formation is taking place. Like, reaction is
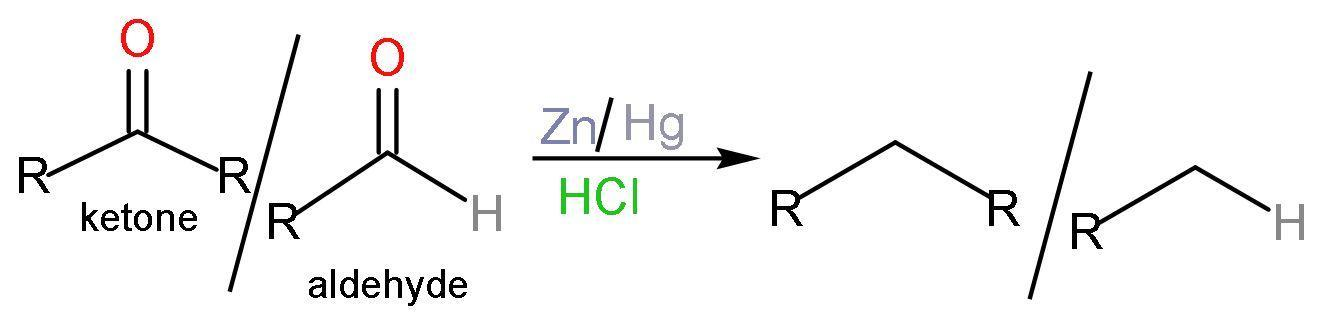
C. Clemmensen reduction: Clemmensen reduction is a chemical reaction in which aldehydes and ketones with zinc amalgam $\left( \text{Zn/Hg} \right)$ , which is an alloy of zinc and mercury in concentrated hydrochloric acid $\left( \text{HCl} \right)$, which reduces the aldehydes and ketones to a hydrocarbon or $\left( -\text{C}{{\text{H}}_{2}}- \right)$ group. No new bond formation is taking place. The reaction is like
D. Reimer-Tiemann reaction: This chemical reaction helps in ortho-formylation of phenols using chloroform and a base. Like, the conversion of phenol to salicylaldehyde. The reaction accounts for the addition of $\left( -\text{CHO} \right)$ group on the benzene ring from the chloroform $\left( \text{CHC}{{\text{l}}_{3}} \right)$ present. The reaction is
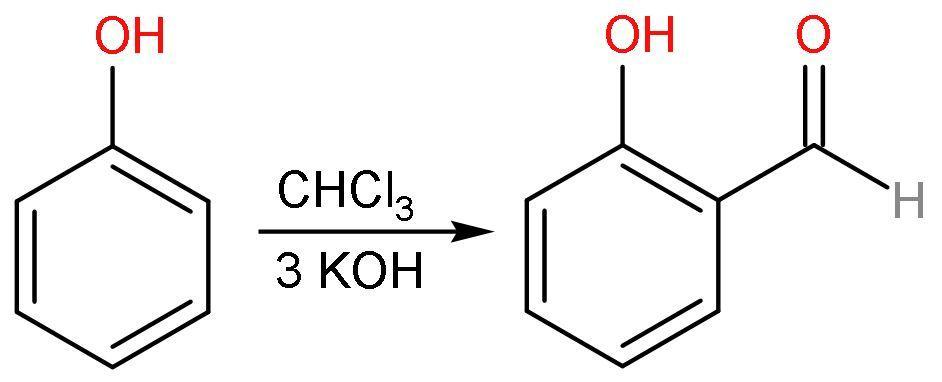
A new $\text{C}-\text{C}$ bond formation is possible in the Reimer-Tiemann reaction.
The correct option is option ‘d’.
Note:
The direct formylation of aromatic compounds can be achieved by various methods like the Gattermann reaction and Gattermann–Koch reaction. However, in terms of ease, the Reimer–Tiemann reaction is the most advantageous reaction over the above reactions, as the Reimer–Tiemann reaction is the only reaction which does not require acidic and anhydrous conditions.
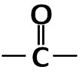
Complete answer:
Let us discuss this question, by discussing the options and there particular reactions:
A. Cannizaro reaction: It is a chemical reaction which involves the disproportionation reaction of two molecules initiated by a base of a non-enolizable (ability to convert to $\text{R}-\text{OH}$) aldehyde to give a carboxylic acid and primary alcohol. No new $\left( \text{C}-\text{C} \right)$ bond formation is taking place. The reaction is

B. Rosenmunds reduction: Rosenmunds reduction reaction is a catalytic hydrogenation process where acyl chlorides are converted into aldehydes by passing hydrogen gas over palladium poisoned by barium sulphate. The barium sulphate reduces the activity of the palladium due to its low surface area, thereby preventing over reduction. No new $\left( \text{C}-\text{C} \right)$ bond formation is taking place. Like, reaction is

C. Clemmensen reduction: Clemmensen reduction is a chemical reaction in which aldehydes and ketones with zinc amalgam $\left( \text{Zn/Hg} \right)$ , which is an alloy of zinc and mercury in concentrated hydrochloric acid $\left( \text{HCl} \right)$, which reduces the aldehydes and ketones to a hydrocarbon or $\left( -\text{C}{{\text{H}}_{2}}- \right)$ group. No new bond formation is taking place. The reaction is like

D. Reimer-Tiemann reaction: This chemical reaction helps in ortho-formylation of phenols using chloroform and a base. Like, the conversion of phenol to salicylaldehyde. The reaction accounts for the addition of $\left( -\text{CHO} \right)$ group on the benzene ring from the chloroform $\left( \text{CHC}{{\text{l}}_{3}} \right)$ present. The reaction is

A new $\text{C}-\text{C}$ bond formation is possible in the Reimer-Tiemann reaction.
The correct option is option ‘d’.
Note:
The direct formylation of aromatic compounds can be achieved by various methods like the Gattermann reaction and Gattermann–Koch reaction. However, in terms of ease, the Reimer–Tiemann reaction is the most advantageous reaction over the above reactions, as the Reimer–Tiemann reaction is the only reaction which does not require acidic and anhydrous conditions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




