
A major component of Borsch reagent is obtained by reacting hydrazine with which of the following?
A.
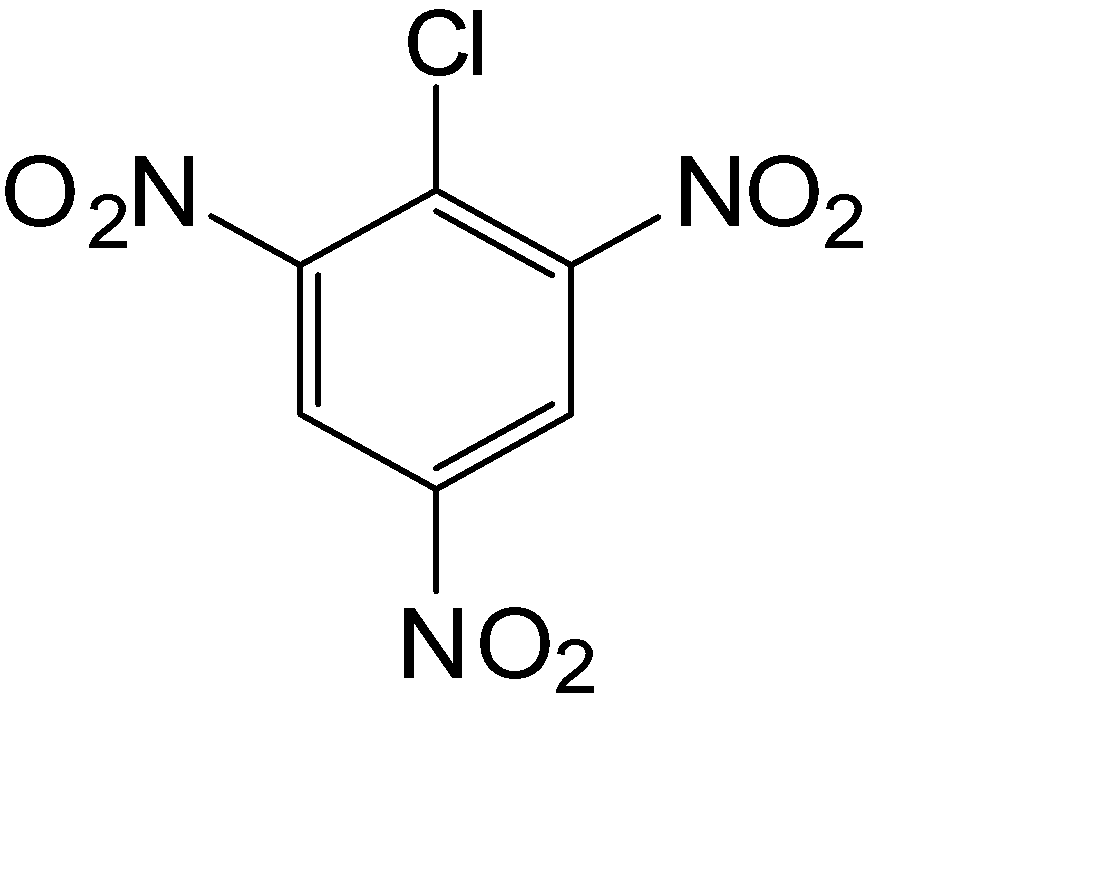
B.
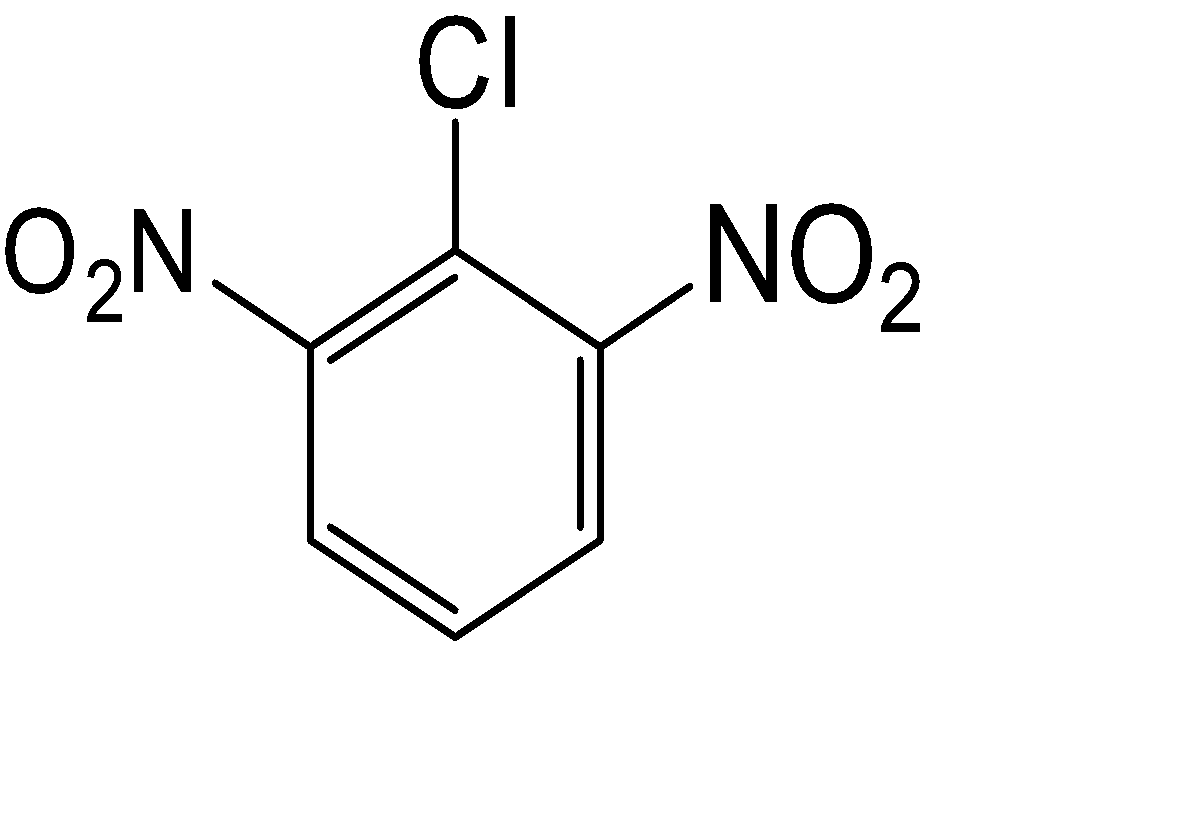
C.
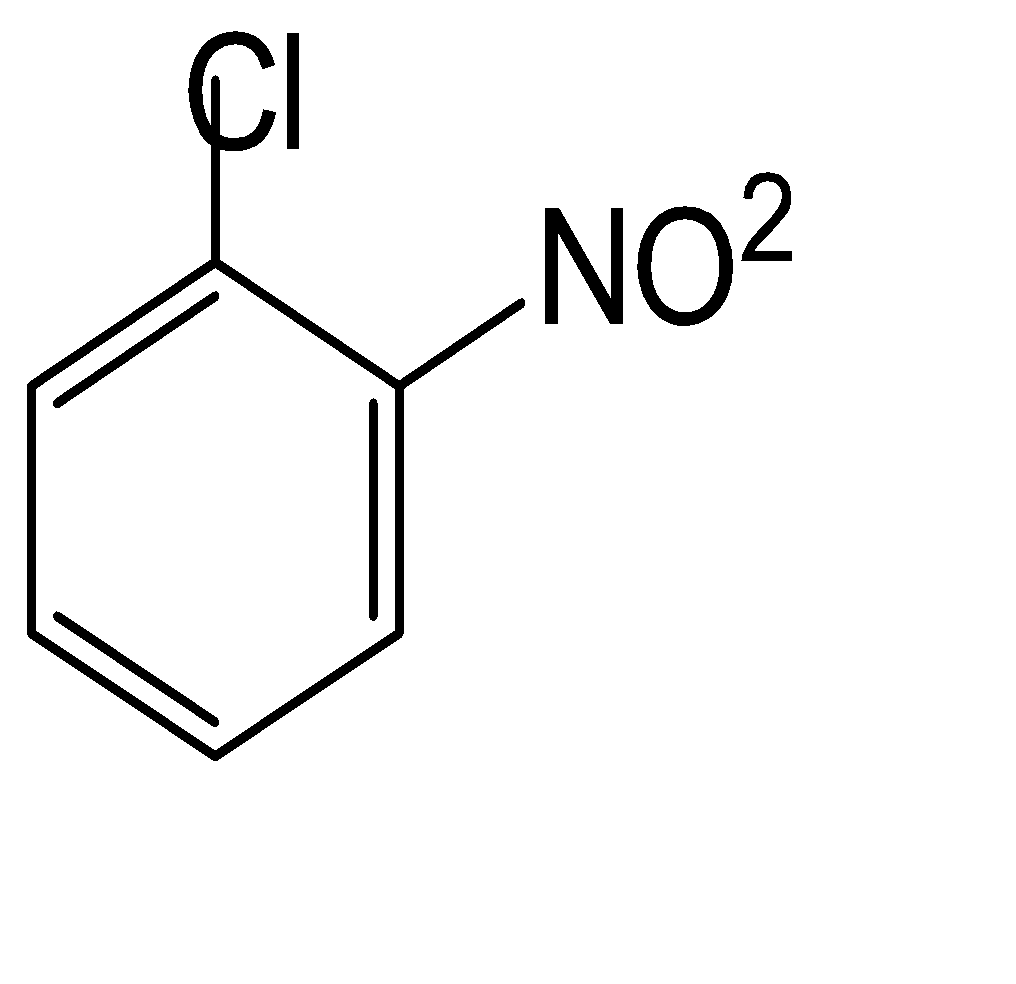
D.
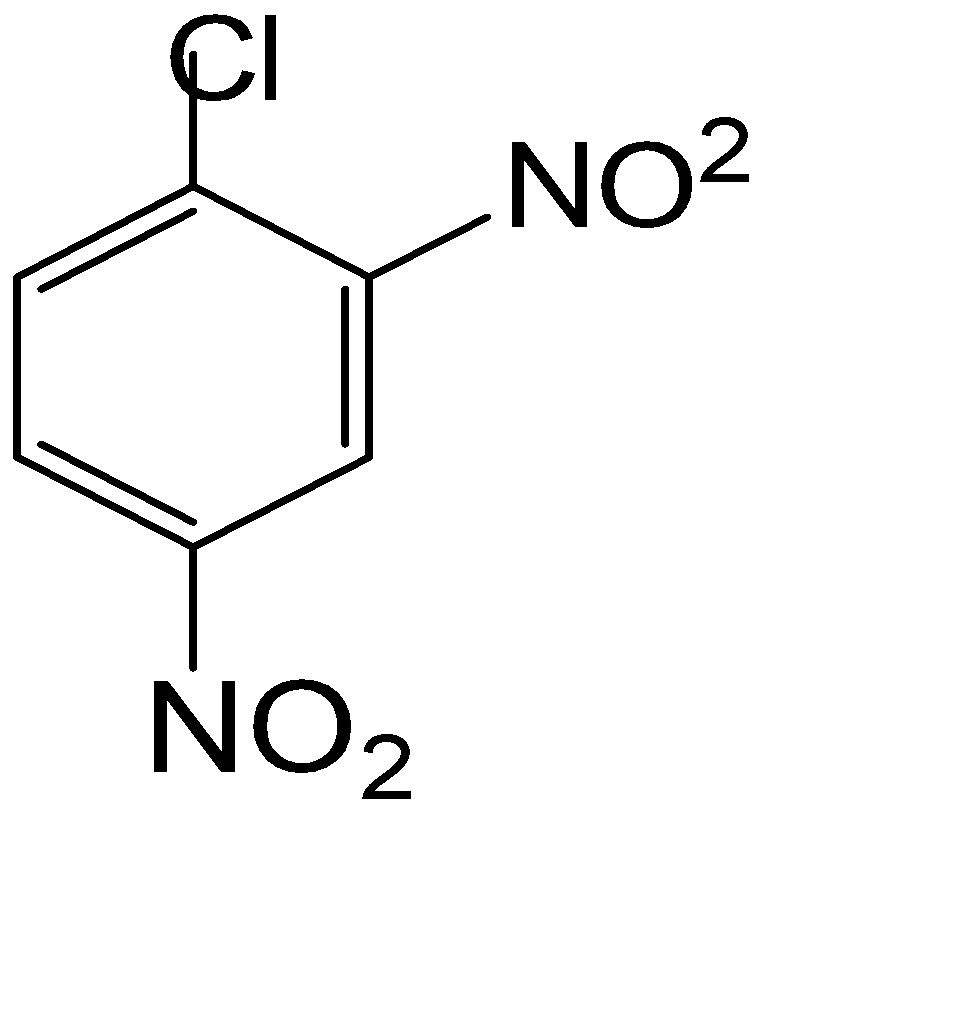




Answer
553.8k+ views
Hint: Benzene is an aromatic non-polar compound which gets attached to by various groups altering its properties and forming new compounds. The various groups being attached to the benzene structure can be electron withdrawing or electron donating which guides the properties of the compound formed and its reactions with various elements and reagents.
Complete step by step answer:
Benzene is an organic planar compound which has the formula of ${C_6}{H_6}$ with 6 carbons attached in the ring in a plane. The electrons in the compounds are delocalized and thus show aromatic properties. The secondary compound that attaches to benzene may be at one of the three positions of ortho, meta, and para. Which are the relative positions with respect to another main substituent already attached to the benzene.
The compound that is attached to benzene in the above question is nitro $( - N{O_2})$ compound and is a strong electron withdrawing in nature. It is also found that nitro group is meta directing means it directs any secondary group to meta position with respect to its position.
2,4-Dinitrophenylhydrazine which is also known as Brady’s reagent and Borsch reagent . It has a chemical formula of \[{C_6}{H_3}{(N{O_2})_2}NHN{H_2}\] . It is a red coloured or orange coloured solid which is a substituted hydrazine. It is also often used to qualitatively test for the carbonyl groups which are linked to the aldehyde and ketonic group.
The compound used for the getting the component of the Borsch reagent is given in option (D)
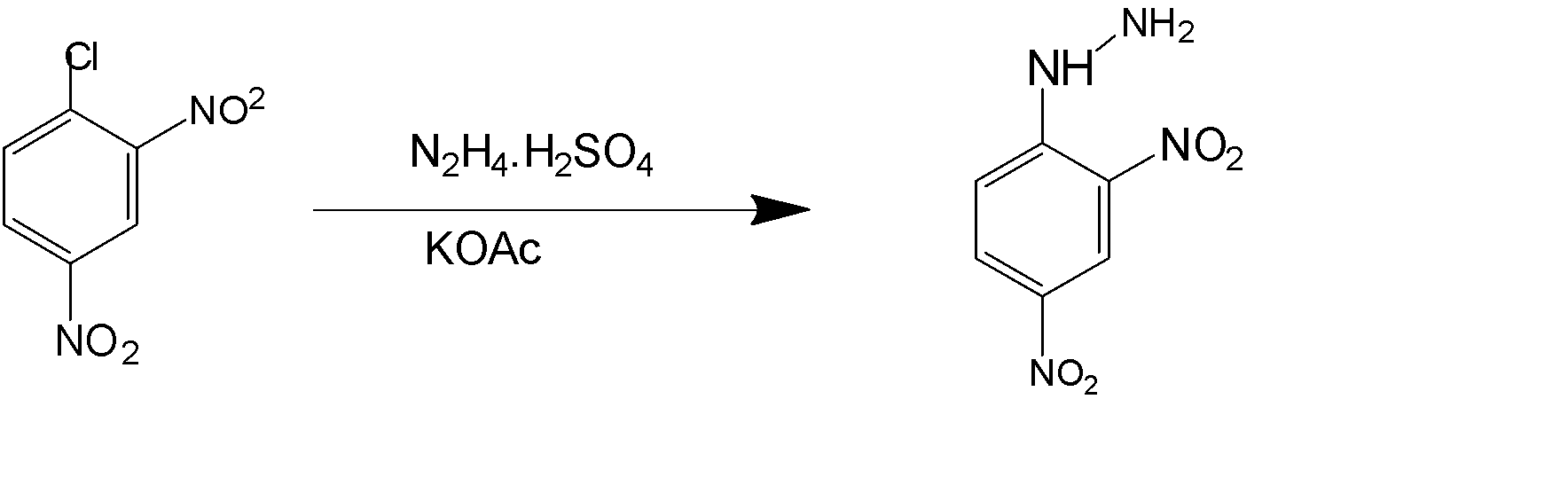
So, the correct answer is Option D.
Note: Hydrazine is an alkaline liquid which is majorly used in chemistry for its powerful reducing properties. It is used in the making of various chemical components and also is a component in the manufacture of certain rocket fuels.
There are various effects that operate in the chemical structure of the compounds .The range from inductive effect, resonance effect, mesomeric effect etc. here $ + M$ is the mesomeric effect. These effects can be positive or negative based on the substituent groups which are attached to the main compound.
Complete step by step answer:
Benzene is an organic planar compound which has the formula of ${C_6}{H_6}$ with 6 carbons attached in the ring in a plane. The electrons in the compounds are delocalized and thus show aromatic properties. The secondary compound that attaches to benzene may be at one of the three positions of ortho, meta, and para. Which are the relative positions with respect to another main substituent already attached to the benzene.
The compound that is attached to benzene in the above question is nitro $( - N{O_2})$ compound and is a strong electron withdrawing in nature. It is also found that nitro group is meta directing means it directs any secondary group to meta position with respect to its position.
2,4-Dinitrophenylhydrazine which is also known as Brady’s reagent and Borsch reagent . It has a chemical formula of \[{C_6}{H_3}{(N{O_2})_2}NHN{H_2}\] . It is a red coloured or orange coloured solid which is a substituted hydrazine. It is also often used to qualitatively test for the carbonyl groups which are linked to the aldehyde and ketonic group.
The compound used for the getting the component of the Borsch reagent is given in option (D)

So, the correct answer is Option D.
Note: Hydrazine is an alkaline liquid which is majorly used in chemistry for its powerful reducing properties. It is used in the making of various chemical components and also is a component in the manufacture of certain rocket fuels.
There are various effects that operate in the chemical structure of the compounds .The range from inductive effect, resonance effect, mesomeric effect etc. here $ + M$ is the mesomeric effect. These effects can be positive or negative based on the substituent groups which are attached to the main compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




