
(a)
(i) Explain Hoffman bromamide degradation for the preparation of Aniline.
(ii) Give the IUPAC name of
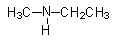 (b) What is Hinsberg reagent ? Between \[C{H_3}N{H_2}\] and \[{C_6}{H_5}N{H_2}\] which is more basic ?
(b) What is Hinsberg reagent ? Between \[C{H_3}N{H_2}\] and \[{C_6}{H_5}N{H_2}\] which is more basic ?
Answer
596.1k+ views
Hint: Hoffman bromamide degradation converts \[ - CON{H_2}\] functional group into \[ - N{H_2}\]. Hinsberg reagent is used in separation of mixtures of amines. As N-atoms get more nucleophilic, its basicity also increases.
Complete step by step solution:
(a) (i)
- Hoffmann bromamide degradation is useful in production of Aniline as it converts the\[ - CON{H_2}\] functional group into the\[ - N{H_2}\]group. So, we need to take a starting material that has the\[ - CON{H_2}\] group and after getting converted into \[ - N{H_2}\] group, the molecule obtained is Aniline. So, in order to obtain Aniline as the final product, we require Benzamide as a starting material. The reaction is as shown below.
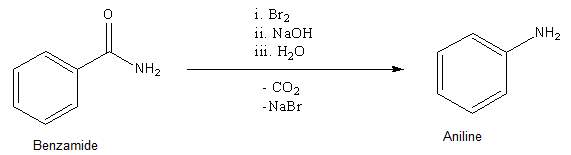
So, in this way, we can easily convert Benzamide to Aniline.
Now, we will try to assign IUPAC names to this compound.
ii)
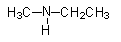
Amines can be named in two ways. We can use the amino word as a prefix and amine word can be used as a suffix as well. Both names are legal according to IUPAC nomenclature.
- First identify the longest alkyl chain, which is of two carbons. There is a substitution of methyl groups on the N-atom. But we will need to specify the place of attachment of the methyl group as N-methyl.
So, its IUPAC name will be N-methylethanamine.
If we use amino prefix, then it will be named as N-methylaminoethane.
(b) Hinsberg reagent is Benzenesulfonyl chloride. Its structure is shown below.
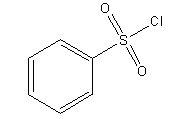
- It is used in separation of mixture of primary, secondary and tertiary amines. Here, atom of amines attack electrophilic S-atoms and undergo reaction.
- Basicity of amines depends upon the ability of atom to donate electrons. Let’s compare basicity of
\[C{H_3}N{H_2}\] and \[{C_6}{H_5}N{H_2}\].
- In Methylamine, \[ - C{H_3}\] is an electron donating group, so it will push electron density towards the N-atom.
- In case of Aniline, N-atom is directly bonded to the benzene ring. So, N-atom is also involved in resonance structures and electron density is not always concentrated on N-atom, so it has comparatively less efficiency to donate electrons than Methyl amine. Also, aromatic rings act as an electron withdrawing group, so that will also reduce electron density on the N-atom.
So, Methyl amine is more basic than Aniline
Additional Information:
- The order of basicity of amines are as follows.
Primary < Secondary < Tertiary
- Hoffman bromamide degradation can be categorized as a rearrangement reaction.
Note: While naming a compound, make sure that you choose the correct parent alkyl chain, otherwise the name will be wrong. Note that Hoffman bromamide degradation involves rearrangement of atoms, which is an exception.
Complete step by step solution:
(a) (i)
- Hoffmann bromamide degradation is useful in production of Aniline as it converts the\[ - CON{H_2}\] functional group into the\[ - N{H_2}\]group. So, we need to take a starting material that has the\[ - CON{H_2}\] group and after getting converted into \[ - N{H_2}\] group, the molecule obtained is Aniline. So, in order to obtain Aniline as the final product, we require Benzamide as a starting material. The reaction is as shown below.
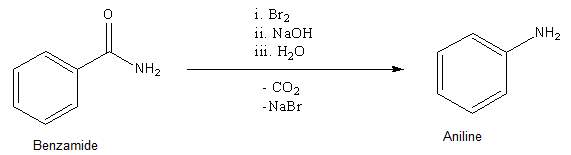
So, in this way, we can easily convert Benzamide to Aniline.
Now, we will try to assign IUPAC names to this compound.
ii)
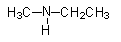
Amines can be named in two ways. We can use the amino word as a prefix and amine word can be used as a suffix as well. Both names are legal according to IUPAC nomenclature.
- First identify the longest alkyl chain, which is of two carbons. There is a substitution of methyl groups on the N-atom. But we will need to specify the place of attachment of the methyl group as N-methyl.
So, its IUPAC name will be N-methylethanamine.
If we use amino prefix, then it will be named as N-methylaminoethane.
(b) Hinsberg reagent is Benzenesulfonyl chloride. Its structure is shown below.

- It is used in separation of mixture of primary, secondary and tertiary amines. Here, atom of amines attack electrophilic S-atoms and undergo reaction.
- Basicity of amines depends upon the ability of atom to donate electrons. Let’s compare basicity of
\[C{H_3}N{H_2}\] and \[{C_6}{H_5}N{H_2}\].
- In Methylamine, \[ - C{H_3}\] is an electron donating group, so it will push electron density towards the N-atom.
- In case of Aniline, N-atom is directly bonded to the benzene ring. So, N-atom is also involved in resonance structures and electron density is not always concentrated on N-atom, so it has comparatively less efficiency to donate electrons than Methyl amine. Also, aromatic rings act as an electron withdrawing group, so that will also reduce electron density on the N-atom.
So, Methyl amine is more basic than Aniline
Additional Information:
- The order of basicity of amines are as follows.
Primary < Secondary < Tertiary
- Hoffman bromamide degradation can be categorized as a rearrangement reaction.
Note: While naming a compound, make sure that you choose the correct parent alkyl chain, otherwise the name will be wrong. Note that Hoffman bromamide degradation involves rearrangement of atoms, which is an exception.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




