
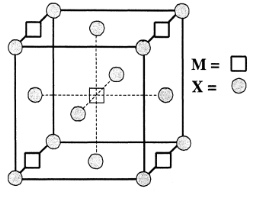
A compound $ {M_p}{X_q} $ has cubic close packing $ (ccp) $ arrangement of X. Its unit cell structure is shown below. The empirical formula of the compound is:
(A) $ MX $
(B) $ M{X_2} $
(C) $ {M_2}X $

Answer
521.4k+ views
Hint :A CCP arrangement has a total of 4 spheres per unit cell and an HCP arrangement has 8 spheres per unit cell. However, both configurations have a coordination number of 12.
The packing efficiency is the fraction of volume in a crystal structure that is occupied by constituent particles, rather than empty space. In order to find this, the volume of the spheres needs to be divided by the total volume (including empty spaces) occupied by the packed spheres.
Complete Step By Step Answer:
If this arrangement has ccp packing then no. of atom of X will be as follows:
The contribution of one corner atom in the unit cell is: $ \dfrac{1}{8} $ atom
So the contribution of all $ 8 $ corner atom will be: $ \dfrac{1}{8} \times 8 = 1atom $
Now the contribution of X type atom of the face of cell
The contribution of one face atom in the unit cell is: $ \dfrac{1}{2}atom $
So the contribution of all $ 6 $ face atom will be: $ \dfrac{1}{2} \times 6 = 3atoms $
Now total number of X atoms will be: $ 1 + 3 = 4atoms $
Now number of M type atoms-
The contribution of one atom on edge is: $ \dfrac{1}{4} $
So the contribution of $ 4 $ edge atoms will be: $ \dfrac{1}{4} \times 4 = 1atom $
Now the contribution of M type atom in the center of unit cell: $ 1atom $
Total number of M type atom will be: $ 1 + 1 = 2atoms $
So, the unit cell formula for the compound will be $ {M_2}{X_4} $ and the empirical formula will be $ M{X_2} $ which is our A option.
Note :
The arrangement of the atoms in a crystalline solid affects atomic coordination numbers, interatomic distances, and the types and strengths of bonding that occur within the solid. An understanding of atomic packing in a unit cell and crystal lattice can give insight into the physical, chemical, electrical, and mechanical properties of a given crystalline material.
The packing efficiency is the fraction of volume in a crystal structure that is occupied by constituent particles, rather than empty space. In order to find this, the volume of the spheres needs to be divided by the total volume (including empty spaces) occupied by the packed spheres.
Complete Step By Step Answer:
If this arrangement has ccp packing then no. of atom of X will be as follows:
The contribution of one corner atom in the unit cell is: $ \dfrac{1}{8} $ atom
So the contribution of all $ 8 $ corner atom will be: $ \dfrac{1}{8} \times 8 = 1atom $
Now the contribution of X type atom of the face of cell
The contribution of one face atom in the unit cell is: $ \dfrac{1}{2}atom $
So the contribution of all $ 6 $ face atom will be: $ \dfrac{1}{2} \times 6 = 3atoms $
Now total number of X atoms will be: $ 1 + 3 = 4atoms $
Now number of M type atoms-
The contribution of one atom on edge is: $ \dfrac{1}{4} $
So the contribution of $ 4 $ edge atoms will be: $ \dfrac{1}{4} \times 4 = 1atom $
Now the contribution of M type atom in the center of unit cell: $ 1atom $
Total number of M type atom will be: $ 1 + 1 = 2atoms $
So, the unit cell formula for the compound will be $ {M_2}{X_4} $ and the empirical formula will be $ M{X_2} $ which is our A option.
Note :
The arrangement of the atoms in a crystalline solid affects atomic coordination numbers, interatomic distances, and the types and strengths of bonding that occur within the solid. An understanding of atomic packing in a unit cell and crystal lattice can give insight into the physical, chemical, electrical, and mechanical properties of a given crystalline material.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




