
A carbonyl compound gives a positive iodoform test but does not reduce Tollen’s reagent or Fehling’s solution. It forms a cyanohydrin with HCN, which on hydrolysis gives a hydroxy acid with a methyl side chain. The compound is :
A) acetaldehyde
B) propionaldehyde
C) acetone
D) crotonaldehyde
Answer
581.4k+ views
Hint: Aldehydes and ketones both are categorized under aldehydes and ketones. The aldehydes can give a positive iodoform test and they can even reduce Tollen’s reagent or Fehling’s solution. The ketones can give positive Iodoform test but they can not reduce Tollens reagent or Fehling’s solution.
Complete step by step solution:
First, let us know what carbonyl compounds are.
The carbonyl compounds include those compounds that contain a $C=O$ bond. Aldehydes and ketones both are classified in carbonyl compounds.
Aldehydes give positive iodoform test and even reduce Tollen’s reagent giving silver mirror test. But ketones give iodoform test positive but they do not reduce Tollens reagent or Fehling’s solution.
So, if we see the options, the option C) acetone is the only ketone while others are aldehydes. So, the options A), B) and D) are ruled out.
Thus, acetone will react with $HCN$ to form cyanohydrin. This on hydrolysis gives hydroxy acid that has a methyl side chain.
The reaction of acetone with $HCN$ is given as -
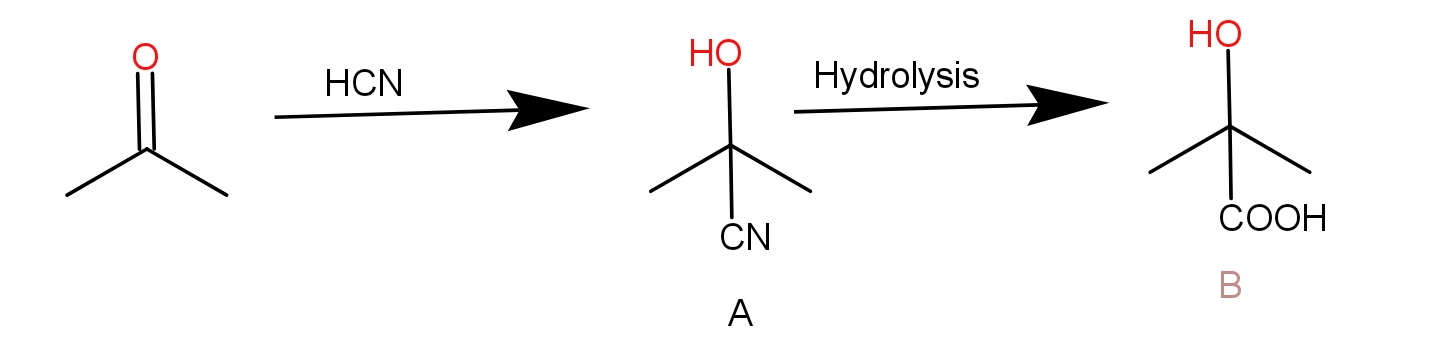
Thus, option C) is the correct answer.
Note: The acetone reacts with HCN to give compound A which is 2 -hydroxy-2-methyl propane nitrile. This is a cyanohydrin. This upon hydrolysis will give compound B which is 2 -hydroxy-2-methyl propanoic acid. The Cyanide is hydrolyzed to give COOH.
Complete step by step solution:
First, let us know what carbonyl compounds are.
The carbonyl compounds include those compounds that contain a $C=O$ bond. Aldehydes and ketones both are classified in carbonyl compounds.
Aldehydes give positive iodoform test and even reduce Tollen’s reagent giving silver mirror test. But ketones give iodoform test positive but they do not reduce Tollens reagent or Fehling’s solution.
So, if we see the options, the option C) acetone is the only ketone while others are aldehydes. So, the options A), B) and D) are ruled out.
Thus, acetone will react with $HCN$ to form cyanohydrin. This on hydrolysis gives hydroxy acid that has a methyl side chain.
The reaction of acetone with $HCN$ is given as -

Thus, option C) is the correct answer.
Note: The acetone reacts with HCN to give compound A which is 2 -hydroxy-2-methyl propane nitrile. This is a cyanohydrin. This upon hydrolysis will give compound B which is 2 -hydroxy-2-methyl propanoic acid. The Cyanide is hydrolyzed to give COOH.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




