
A carbohydrate X having a molecular weight of 180 g/mol has one primary alcohol group and four secondary alcohol groups. It reacts with acetic anhydride to form pentaacetate. The molecular weight of pentaacetate formed is:
(A) 180
(B) 210
(C) 390
(D) 42
Answer
582k+ views
Hint: To solve this firstly you have to use the number of primary and secondary alcohols given in the question. Do not forget that carbohydrates contain aldehydes or ketone groups so determine that too. From that structure find out the product formed when it reacts with acetic anhydride and then find out its molecular weight.
Complete step by step solution:
To answer this question, we have to understand what carbohydrates are and also about primary and secondary alcohols.
Carbohydrates are basically hydrocarbons and they also contain oxygen atoms where the ratio of hydrogen to oxygen is 2:1.
Then we have primary and secondary alcohols.
A primary alcohol is a compound with the –OH group attached to the primary carbon atom. In a primary carbon atom, all the other groups are hydrogen other than the –OH group. We can represent it as $C{{H}_{2}}OH$.
Secondary alcohol is a compound where the carbon atom is bonded to a hydroxyl group, hydrogen atom and one alkyl group. Basically, if we replace a hydrogen atom from the primary alcohol by an alkyl group, it will give us secondary alcohol. The general formula is $CHROH$.
Now, let us discuss the question given to us.
Here the carbohydrate contains one primary alcohol i.e. one group is $C{{H}_{2}}OH$and 4 secondary alcohols. Also, if we calculate its molar mass it should come out to be 180.
Therefore, we can write down its structure as-
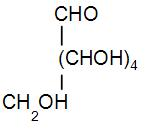
We identify this structure as glucose. When it reacts with acetic anhydride, it gives penta-acetyl glucose and acetic acid. We can write the reaction as-
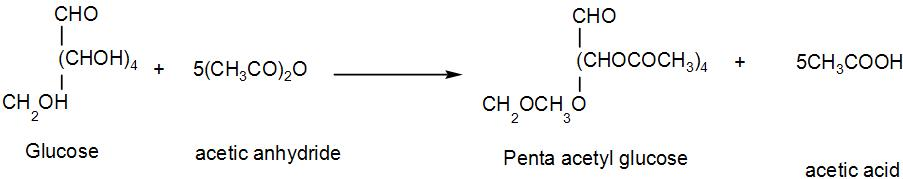
Now we can calculate the molecular weight of the pentaacetate formed from here.
Here we have 16 carbon atoms, 22 hydrogen atoms and 11 oxygen atoms.
The atomic weight of carbon, hydrogen and oxygen is 12, 1 and 16 respectively.
So, molecular weight of the penta-acetate is $\left( 12\times 16 \right)+\left( 22\times 1 \right)+\left( 16\times 11 \right)$ = 390 g/mol
Therefore, the correct answer is option [C] 390.
Note: Alcohols are known to be primary, secondary or tertiary depending upon the nature of carbon atom the hydroxyl group is bonded to. We have already discussed primary and secondary alcohols above and tertiary alcohol is a compound where there are no hydrogen substituents on the carbon atom. Its general formula is $C{{R}_{2}}OH$.
Glucose is basically a sugar which is a carbohydrate and its structure is-
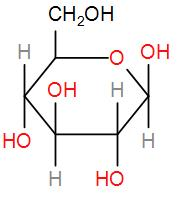
Complete step by step solution:
To answer this question, we have to understand what carbohydrates are and also about primary and secondary alcohols.
Carbohydrates are basically hydrocarbons and they also contain oxygen atoms where the ratio of hydrogen to oxygen is 2:1.
Then we have primary and secondary alcohols.
A primary alcohol is a compound with the –OH group attached to the primary carbon atom. In a primary carbon atom, all the other groups are hydrogen other than the –OH group. We can represent it as $C{{H}_{2}}OH$.
Secondary alcohol is a compound where the carbon atom is bonded to a hydroxyl group, hydrogen atom and one alkyl group. Basically, if we replace a hydrogen atom from the primary alcohol by an alkyl group, it will give us secondary alcohol. The general formula is $CHROH$.
Now, let us discuss the question given to us.
Here the carbohydrate contains one primary alcohol i.e. one group is $C{{H}_{2}}OH$and 4 secondary alcohols. Also, if we calculate its molar mass it should come out to be 180.
Therefore, we can write down its structure as-
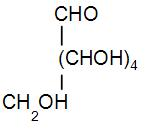
We identify this structure as glucose. When it reacts with acetic anhydride, it gives penta-acetyl glucose and acetic acid. We can write the reaction as-

Now we can calculate the molecular weight of the pentaacetate formed from here.
Here we have 16 carbon atoms, 22 hydrogen atoms and 11 oxygen atoms.
The atomic weight of carbon, hydrogen and oxygen is 12, 1 and 16 respectively.
So, molecular weight of the penta-acetate is $\left( 12\times 16 \right)+\left( 22\times 1 \right)+\left( 16\times 11 \right)$ = 390 g/mol
Therefore, the correct answer is option [C] 390.
Note: Alcohols are known to be primary, secondary or tertiary depending upon the nature of carbon atom the hydroxyl group is bonded to. We have already discussed primary and secondary alcohols above and tertiary alcohol is a compound where there are no hydrogen substituents on the carbon atom. Its general formula is $C{{R}_{2}}OH$.
Glucose is basically a sugar which is a carbohydrate and its structure is-

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




