
A by-product of soap industry is:
A. Sodium hydroxide
B. Sodium palmitate
C. Glycerol
D. Both B and C
Answer
597.3k+ views
Hint: To answer the by-product of the soap industry, we should know that by-product is a secondary product derived from a production process. And the answer of this solution is viscous liquid that is sweet-tasting and non-toxic.
Step by step answer:
So, first of all we should understand what saponification is. In common terms we can say that the process by which we make soap is called saponification. This is the definition in common language. We know that soap is now an essential everyday item and finds its importance in everyday life. We use soaps from cleaning ourselves to cleaning everything around us.
So, from the above paragraph we know that saponification is simply the process of making soaps. We should note that soaps are just potassium or sodium salts of long-chain fatty acids. During saponification, ester reacts with an inorganic base to produce alcohol and soap.
The fat reacts with NaOH or KOH to give by-product of glycerol and sodium or potassium salt of the fatty acid. This reaction is represented below:
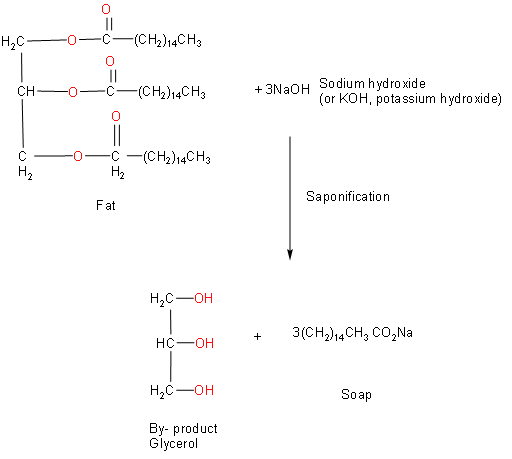
From the above reaction, we can say that Glycerol is the by-product of soap industries.Soaps are sodium or potassium salts of fatty acids, made by boiling together with potash (potassium hydroxide). Hydrolysis of the fats and oils occurs; yielding glycerol and crude soap.by-product in the production of soap from fat is glycerol. So, from this we can say that option C is correct.
Note: If you ever get the opportunity to visit an exhibition of old oil painting, you will observe the damage on it. Saponification can occur in oil paintings over time, causing visible damage and deformation. The oil paints that we use in painting are composed of pigment molecules suspended in an oil binding medium. We use heavy metals for imparting colour pigments and if these heavy metals react with free fatty acids in the oil medium, metal soaps may form in a paint layer that can then migrate outward to the painting's surface.
Step by step answer:
So, first of all we should understand what saponification is. In common terms we can say that the process by which we make soap is called saponification. This is the definition in common language. We know that soap is now an essential everyday item and finds its importance in everyday life. We use soaps from cleaning ourselves to cleaning everything around us.
So, from the above paragraph we know that saponification is simply the process of making soaps. We should note that soaps are just potassium or sodium salts of long-chain fatty acids. During saponification, ester reacts with an inorganic base to produce alcohol and soap.
The fat reacts with NaOH or KOH to give by-product of glycerol and sodium or potassium salt of the fatty acid. This reaction is represented below:

From the above reaction, we can say that Glycerol is the by-product of soap industries.Soaps are sodium or potassium salts of fatty acids, made by boiling together with potash (potassium hydroxide). Hydrolysis of the fats and oils occurs; yielding glycerol and crude soap.by-product in the production of soap from fat is glycerol. So, from this we can say that option C is correct.
Note: If you ever get the opportunity to visit an exhibition of old oil painting, you will observe the damage on it. Saponification can occur in oil paintings over time, causing visible damage and deformation. The oil paints that we use in painting are composed of pigment molecules suspended in an oil binding medium. We use heavy metals for imparting colour pigments and if these heavy metals react with free fatty acids in the oil medium, metal soaps may form in a paint layer that can then migrate outward to the painting's surface.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




