
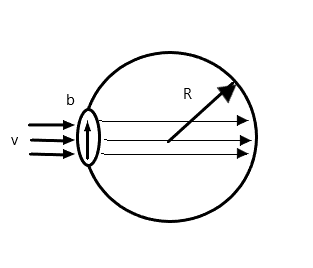
A bubble having surface tension $T$ and radius $R$ is formed on a ring of radius $b$ ($b < R$). Air is blown inside the tube with velocity $v$ as shown. The air molecule collides perpendicularly with the wall of the bubble and stops. The radius at which the bubble separates from the ring is
(A) $\dfrac{{2T}}{{\rho {v^2}}}$
(B) $\dfrac{{2T}}{{\rho v}}$
(C) $\dfrac{{4T}}{{\rho {v^2}}}$
(D) $\dfrac{{4T}}{{\rho v}}$

Answer
581.1k+ views
Hint For bubble to form beyond ring at a radius $b$ greater than $R$, then excess pressure must exist within the bubble. Because there is no acceleration of the molecule, then the force of the air must be equal to the force due to excess pressure within the bubble. On equating the formulas we get the solution.
Formula used In the solution we will be using the following formulas,
$\Rightarrow P = \dfrac{{4T}}{R}$ where $P$ is the excess pressure within the bubble, $T$ is the surface tension, and $R$ is the radius of the bubble.
Force exerted by air molecules ${F_a} = \rho A{v^2}$ where $\rho $ is the density of air, $A$ is the area on which the force is exerted on, and $v$ is the speed of the air.
Complete step by step answer
To solve the above equation, we note the bubble must contain an excess pressure within it to expand outwards. The excess pressure in a bubble is given by
$\Rightarrow P = \dfrac{{4T}}{R}$ where, $T$ is the surface tension and $R$ is the radius of the bubble.
This pressure pushes outward from the inside of the bubble.
According to the question, air collides with the bubble. These air molecules must exert a force on the bubble.
The force exerted by the molecules is given by
$\Rightarrow {F_a} = \rho A{v^2}$where$\rho $ is the density of air, $A$ is the area the force exerted on, and $v$ is the speed of the air.
Now, since there’s no acceleration of the bubble, the force exerted due to the excess pressure inside the bubble is equal to the force exerted by the air molecules hence on equating we get,
$\Rightarrow {F_a} = PA \Rightarrow \rho A{v^2} = \dfrac{{4AT}}{R}$ (Force due to excess pressure is $PA$. )
Making $R$ subject of the formula and cancelling the like terms, we have
$\Rightarrow R = \dfrac{{4T}}{{\rho {v^2}}}$
Hence, the correct option is (C).
Note
Alternatively, we can eliminate option B and D using a unit check. The unit of surface tension is N/m. Hence on substituting the units of the variables in the answers given for option B and D we have
$\Rightarrow \dfrac{N}{m} \div \left( {\dfrac{{kg}}{{{m^3}}} \times m{s^{ - 1}}} \right)$
$\Rightarrow \dfrac{{kgm{s^{ - 2}}}}{m} \times \left( {\dfrac{{{m^2}}}{{kg{s^{ - 1}}}}} \right) = {m^2}{s^{ - 1}}$ This is not the unit of distance.
Also from knowledge that
$\Rightarrow P = \dfrac{{4T}}{R}$ since force exerted by air is a dimensionless constant, we can safely say that option C is the solution.
Formula used In the solution we will be using the following formulas,
$\Rightarrow P = \dfrac{{4T}}{R}$ where $P$ is the excess pressure within the bubble, $T$ is the surface tension, and $R$ is the radius of the bubble.
Force exerted by air molecules ${F_a} = \rho A{v^2}$ where $\rho $ is the density of air, $A$ is the area on which the force is exerted on, and $v$ is the speed of the air.
Complete step by step answer
To solve the above equation, we note the bubble must contain an excess pressure within it to expand outwards. The excess pressure in a bubble is given by
$\Rightarrow P = \dfrac{{4T}}{R}$ where, $T$ is the surface tension and $R$ is the radius of the bubble.
This pressure pushes outward from the inside of the bubble.
According to the question, air collides with the bubble. These air molecules must exert a force on the bubble.
The force exerted by the molecules is given by
$\Rightarrow {F_a} = \rho A{v^2}$where$\rho $ is the density of air, $A$ is the area the force exerted on, and $v$ is the speed of the air.
Now, since there’s no acceleration of the bubble, the force exerted due to the excess pressure inside the bubble is equal to the force exerted by the air molecules hence on equating we get,
$\Rightarrow {F_a} = PA \Rightarrow \rho A{v^2} = \dfrac{{4AT}}{R}$ (Force due to excess pressure is $PA$. )
Making $R$ subject of the formula and cancelling the like terms, we have
$\Rightarrow R = \dfrac{{4T}}{{\rho {v^2}}}$
Hence, the correct option is (C).
Note
Alternatively, we can eliminate option B and D using a unit check. The unit of surface tension is N/m. Hence on substituting the units of the variables in the answers given for option B and D we have
$\Rightarrow \dfrac{N}{m} \div \left( {\dfrac{{kg}}{{{m^3}}} \times m{s^{ - 1}}} \right)$
$\Rightarrow \dfrac{{kgm{s^{ - 2}}}}{m} \times \left( {\dfrac{{{m^2}}}{{kg{s^{ - 1}}}}} \right) = {m^2}{s^{ - 1}}$ This is not the unit of distance.
Also from knowledge that
$\Rightarrow P = \dfrac{{4T}}{R}$ since force exerted by air is a dimensionless constant, we can safely say that option C is the solution.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




