
How many 3C - 4${{\text{e}}^{-}}$ bonds are present in the dimer of $\text{BeC}{{\text{l}}_{2}}$?
Answer
600k+ views
Hint: 3C-4${{\text{e}}^{-}}$ bonds stands for three centre - 4 electron bonds. Coordination bonds are those bonds which are made after the sharing of lone pair electrons. 3C-4${{\text{e}}^{-}}$ bond means that in between 3 atoms total 4 electrons are shared which do not participate in the reaction.
Complete answer:
-Firstly, we have to draw the structure of beryllium chloride.
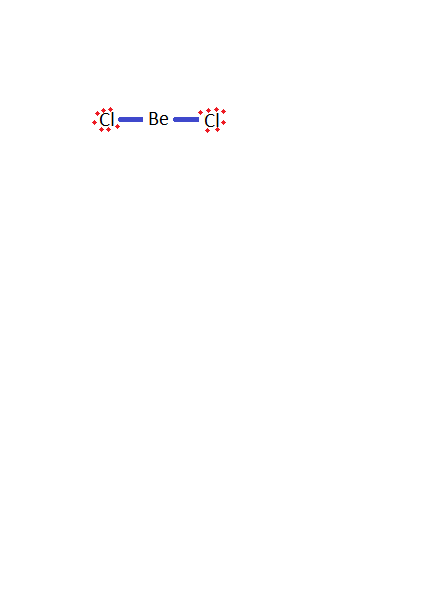
-Now, here chlorine has a lone pair of an electron which can be shared with the other molecules which are electron deficient.
-So, beryllium chloride dimerises to form \[\text{B}{{\text{e}}_{2}}\text{C}{{\text{l}}_{4}}\] because it is unstable due to incomplete octet present in the boron and the structure of it is:
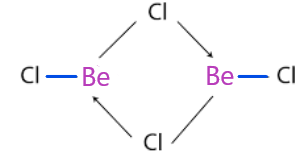
-So, we can see that the two molecules of chlorine share the lone pair of an electron with boron to fulfill the octet.
-Also, the compound is an electron-deficient molecule because electron-deficient molecules are those in which the total no. of atoms in the boron is less than 8.
-As we can also see that four-electrons are being shared, that is two electrons from each chlorine atom and they are given to the boron.
-So, a total of 3 atoms participated that's why it is called 3 centre - 4 electron bond.
-And a total of two bonds are formed.
Therefore, total 2, 3C - 4${{\text{e}}^{-}}$ bonds in the structure of beryllium chloride.
Note: Electron deficient molecules tend to accept electrons so they can make coordinate bonds easily. Other molecules which can form 3C - 4${{\text{e}}^{-}}$bonds are \[\text{Xe}{{\text{F}}_{2}}\text{, A}{{\text{l}}_{2}}\text{C}{{\text{l}}_{6}}\text{, HF, etc}\]. Electron rich molecules are those species which have an extra pair of electrons to make the bond with electron deficient molecules.
Complete answer:
-Firstly, we have to draw the structure of beryllium chloride.

-Now, here chlorine has a lone pair of an electron which can be shared with the other molecules which are electron deficient.
-So, beryllium chloride dimerises to form \[\text{B}{{\text{e}}_{2}}\text{C}{{\text{l}}_{4}}\] because it is unstable due to incomplete octet present in the boron and the structure of it is:

-So, we can see that the two molecules of chlorine share the lone pair of an electron with boron to fulfill the octet.
-Also, the compound is an electron-deficient molecule because electron-deficient molecules are those in which the total no. of atoms in the boron is less than 8.
-As we can also see that four-electrons are being shared, that is two electrons from each chlorine atom and they are given to the boron.
-So, a total of 3 atoms participated that's why it is called 3 centre - 4 electron bond.
-And a total of two bonds are formed.
Therefore, total 2, 3C - 4${{\text{e}}^{-}}$ bonds in the structure of beryllium chloride.
Note: Electron deficient molecules tend to accept electrons so they can make coordinate bonds easily. Other molecules which can form 3C - 4${{\text{e}}^{-}}$bonds are \[\text{Xe}{{\text{F}}_{2}}\text{, A}{{\text{l}}_{2}}\text{C}{{\text{l}}_{6}}\text{, HF, etc}\]. Electron rich molecules are those species which have an extra pair of electrons to make the bond with electron deficient molecules.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




