
2-phenylethanol may be prepared by the reaction of phenylmagnesium bromide with:
A. HCHO
B. $C{{H}_{3}}CHO$
C. $C{{H}_{3}}COC{{H}_{3}}$
D.
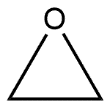

Answer
577.8k+ views
Hint: 2-phenylethanol can also be known by the name phenethyl alcohol. It is generally an organic compound consists a phenethyl group attached with alcohol and show as ${{C}_{6}}{{H}_{5}}C{{H}_{2}}C{{H}_{2}}OH$. It is a colorless liquid and has less solubility in water as compared with organic solvents.
Complete Step by step solution: Phenyl magnesium bromide is represented by ${{C}_{6}}{{H}_{5}}MgBr$ it is kept in the category of organometallic compounds. It is known as Grignard reagent. It is a strong nucleophile as well as a strong base. It can easily abstract acidic protons. With carbon dioxide phenyl magnesium bromide reacts to give benzoic acid after an acidic workup. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF).
-Given reaction is an example of nucleophilic reaction; these are those chemical addition reactions in which a nucleophile forms a sigma bond with an electron deficient species. In this reaction nucleophilic attack is done on epoxide ring which opens the epoxide ring and the reaction can be shown as follows:
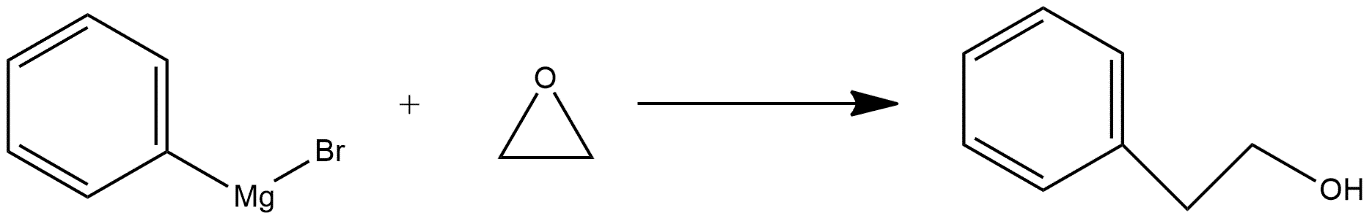
Hence we can say that option D is the correct answer.
Note: Grignard reagents are one of the most useful organometallic compounds. They exhibit strong nucleophilic qualities and also have the ability to form new carbon-carbon bonds; it is generally represented as an organomagnesium compound with the chemical formula R-MgX where R may be any alkyl or aryl group while X is halogen.
Complete Step by step solution: Phenyl magnesium bromide is represented by ${{C}_{6}}{{H}_{5}}MgBr$ it is kept in the category of organometallic compounds. It is known as Grignard reagent. It is a strong nucleophile as well as a strong base. It can easily abstract acidic protons. With carbon dioxide phenyl magnesium bromide reacts to give benzoic acid after an acidic workup. It is commercially available as a solution in diethyl ether or tetrahydrofuran (THF).
-Given reaction is an example of nucleophilic reaction; these are those chemical addition reactions in which a nucleophile forms a sigma bond with an electron deficient species. In this reaction nucleophilic attack is done on epoxide ring which opens the epoxide ring and the reaction can be shown as follows:
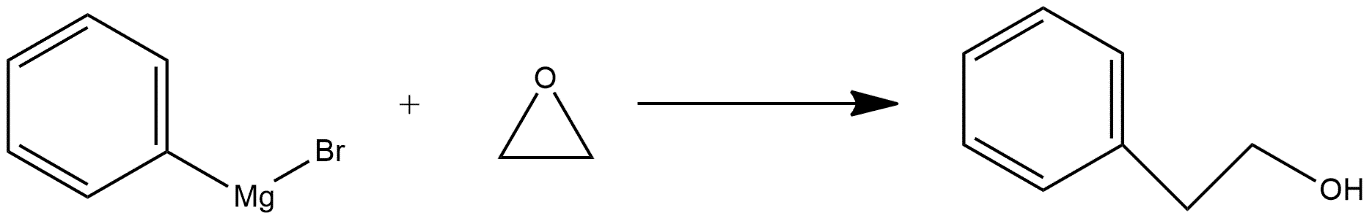
Hence we can say that option D is the correct answer.
Note: Grignard reagents are one of the most useful organometallic compounds. They exhibit strong nucleophilic qualities and also have the ability to form new carbon-carbon bonds; it is generally represented as an organomagnesium compound with the chemical formula R-MgX where R may be any alkyl or aryl group while X is halogen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




