How Can You Use Chemical Bonding And Molecular Structure Class 11 NCERT PDF To Prepare Better For Exams
FAQs on NCERT Solutions For Class 11 Chemistry Chapter 4 Chemical Bonding And Molecular Structure - 2025-26
1. What is the octet rule and why does it sometimes fail as per NCERT Solutions for Class 11 Chemistry Chapter 4?
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight electrons in their valence shell, similar to noble gases. However, this rule fails for molecules involving elements from period 3 or higher (which can expand their octet), those with incomplete octets (like BeCl₂), odd-electron species (like NO), and compounds involving noble gases or large differences in atom sizes. As per CBSE 2025–26, understanding these exceptions is essential for mastering the NCERT Solutions for Class 11 Chemistry Chapter 4.
2. How do you represent ions and molecules using Lewis dot structures as required in Chapter 4 NCERT Solutions?
Lewis dot structures use dots to represent valence electrons around element symbols. For ions, adjust the total number of electrons for charge and enclose the structure in brackets with the charge outside (e.g., [ :Ö:: ]2− for O2−). For molecules, show all bonding and lone pairs to visualize electron sharing or transfer. Practicing these diagrams strengthens your answers as per the Class 11 Chemistry NCERT Solutions methodology.
3. What is the VSEPR theory and how does it help predict molecular shape in NCERT Solutions for Class 11 Chemistry Chapter 4?
Valence Shell Electron Pair Repulsion (VSEPR) theory explains molecular shapes by stating that electron pairs (bonding and lone) around a central atom repel each other and arrange to minimize this repulsion. Thus, the geometry (linear, trigonal planar, tetrahedral, etc.) depends on the count of bonding and lone pairs. Applying VSEPR is a key skill as per the NCERT Solutions for Class 11 Chemistry Chapter 4 guidelines for CBSE 2025–26.
4. How is bond order determined using Molecular Orbital Theory according to the NCERT Class 11 Chemistry Chapter 4 Solutions?
Bond order is calculated as half the difference between the number of bonding and antibonding electrons: Bond order = ½ (number of bonding electrons − number of antibonding electrons). A higher bond order signifies stronger and shorter bonds. For example, N₂ has a bond order of 3, O₂ has 2; molecules with zero or negative bond order are unstable and don't exist under normal conditions.
5. Why is BeH₂ linear even though the Be–H bonds are polar? (Frequently updated for CBSE 2025–26 exams)
BeH₂ is linear because the beryllium atom forms two equivalent bonds with hydrogen, and there are no lone pairs on the central atom. The molecule thus arranges the bonds at 180° (linear). Though Be–H bonds are individually polar, the dipoles are equal and opposite, so the net dipole moment is zero, leading to a nonpolar molecule overall.
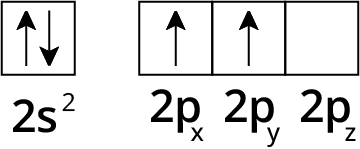
6. What is hybridization and how do sp, sp², and sp³ hybrid orbitals differ according to NCERT Solutions?
Hybridization is the mixing of atomic orbitals to form new hybrid orbitals of equal energy.
- sp hybridization: forms two orbitals, linear geometry (180°), e.g., BeCl₂
- sp² hybridization: forms three orbitals, trigonal planar geometry (120°), e.g., BF₃
- sp³ hybridization: forms four orbitals, tetrahedral geometry (109.5°), e.g., CH₄
7. How is the strength of a bond expressed in relation to bond order in Class 11 Chemistry Chapter 4 NCERT Solutions?
The strength of a bond is directly related to bond order: the higher the bond order, the greater the bond strength and bond energy. For example, a triple bond (bond order 3) in N₂ is stronger than a double bond (bond order 2) in O₂.
8. What are resonance structures and why are they important for molecules like SO₃ and CO₃²⁻?
Resonance structures are alternative Lewis structures for the same molecule showing possible arrangements of electrons, but without moving atoms. They are important when a single Lewis structure fails to describe all observed properties (like bond lengths). The true structure is a resonance hybrid, which is more stable than any single canonical form (key for CO₃²⁻, SO₃ in CBSE syllabus).
9. What are the most common misconceptions about chemical bonding asked in Class 11 NCERT Solutions and how should students avoid them?
Common misconceptions include:
- Thinking all molecules follow the octet rule (exceptions must be learned, e.g., PF₅, NO, BeCl₂)
- Assuming all polar bonds make a polar molecule (molecular geometry can cause dipole cancellation)
- Misapplying VSEPR by not counting lone pairs accurately
10. How do you differentiate between sigma (σ) and pi (π) bonds as per NCERT Class 11 Chemistry Chapter 4 Solutions?
Sigma (σ) bonds form by head-on overlap of orbitals, are stronger, and allow free rotation. Pi (π) bonds form by sideways overlap of p orbitals, are weaker, and restrict rotation. Single bonds are always sigma; multiple bonds include one sigma and the rest pi bonds.
11. What is the significance of dipole moment in predicting the shape and polarity of molecules in Chemical Bonding and Molecular Structure NCERT Solutions?
Dipole moment quantifies the charge separation in a molecule, indicating its polarity. Molecules with nonzero dipole moments are polar; molecules with symmetrical geometry (like CO₂) can be nonpolar despite individual polar bonds, as their dipoles cancel. Dipole moment helps predict molecular geometry and physical properties.
12. How does electron gain enthalpy differ from electronegativity according to the NCERT Solutions for Class 11 Chemistry Chapter 4?
Electron gain enthalpy is the energy change when an electron is added to a neutral atom in the gaseous state, measured in kilojoules per mole. Electronegativity is the relative tendency of an atom to attract electrons in a chemical bond; it is unitless and not directly measurable. Electron gain enthalpy is a property of isolated atoms, while electronegativity also depends on bonding context.
13. Why does NH₃ have a greater dipole moment than NF₃, despite fluorine being more electronegative than hydrogen? (Key for CBSE 2025–26)
Though fluorine is more electronegative, in NH₃ the bond dipoles and lone pair dipole are additive, resulting in a higher net dipole moment (1.46 D). In NF₃, the highly electronegative fluorines orient the bond dipoles opposite to the lone pair, causing partial cancellation and a much lower net dipole moment (0.24 D). This is a frequent exam trap covered in NCERT Solutions Chapter 4.
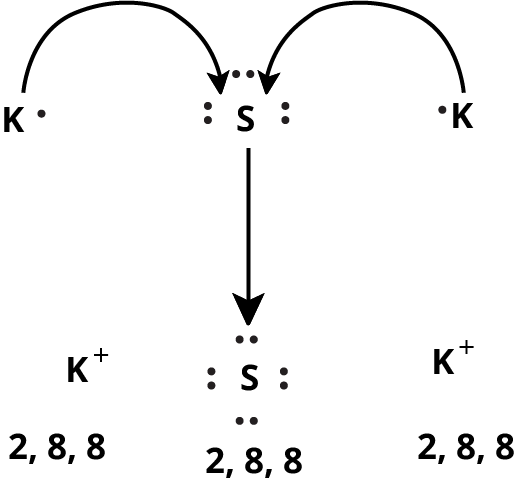
14. What are the favourable conditions for the formation of ionic bonds as per NCERT Class 11 Chemistry Chapter 4 guidelines?
Favourable conditions for ionic bond formation include:
- Low ionization energy of the metal (easy electron loss)
- High electron gain enthalpy of the nonmetal (easy electron gain)
- High lattice energy of the resulting ionic compound (stabilizes ions)
15. Explain the difference between bond length and bond angle with examples from Class 11 Chemistry Chapter 4 NCERT Solutions.
Bond length is the average equilibrium distance between the nuclei of two bonded atoms (e.g., 74 pm for H₂). Bond angle is the angle between two bonds at the same atom (e.g., 104.5° in H₂O). Both parameters help determine the geometry and physical properties of molecules in the CBSE syllabus.