




Key Properties and Interesting Facts About Chlorine
Have you ever thought about what chlorine is while studying the periodic table in your science class? Well, it is a chemical element that is present in a gaseous state in the environment. It is greenish yellow and is heavier than air. Scientists have given the symbol of chlorine, Cl. They have also defined Cl atomic number as 17. The atomic number is the number of a chemical element in the periodic table.

Chlorine Symbol and Atomic Number
Chlorine is poisonous to humans, especially to our eyes and respiratory system. However, small amounts of it are used to disinfect water for drinking. Besides, it is used for several other purposes across various industries.
Where is Chlorine found?
Majorly, chlorine exists in the form of gas and is found with other gases in nature after any explosion or volcanic eruption. However, it also exists in a solid or liquid state in combination with other chemical elements. The common example of chlorine in the solid state is common salt or sodium chloride, whereas, in the liquid state, it is hydrochloric acid, a juice found in the human stomach which helps in digestion.

Solid Chlorine
What are the Properties of Chlorine?
Chlorine shows different physical and chemical properties due to its atomic structure which also defines Cl atomic number. Let us discuss these properties in detail.
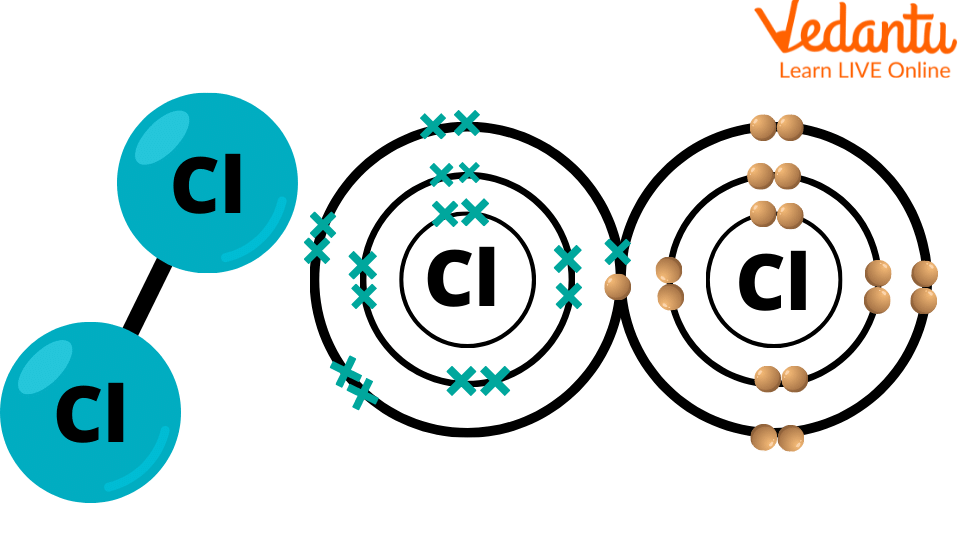
Chlorine Structure
Physical Properties
The physical properties of chlorine are as follows;
Chlorine is a greenish yellow gas.
It has a suffocating or strong smell.
It has a melting point of 171.6 K and a boiling point of 239.11 K.
It is 2 to 5 times denser than air.
It can be condensed or converted into liquid or solid.
It is less dissolvable in water.
It exists in di-molecule form (two-molecule) Cl2.
The symbol of chlorine is Cl.
The atomic number of chlorine is 17.
Chemical Properties
The chemical properties of chlorine are;
Chlorine, when added to water, gives chlorine water which is a yellow and firm-smelling liquid. The water's colour disappears in daylight.
Hypochlorous acid, a chlorine liquid compound, releases oxygen when it breaks during a chemical reaction. This oxygen is used for bleaching clothes.
It combines with non-metals except carbon, oxygen and nitrogen.
It is reactive to metals and forms compounds. These compounds are called chlorides.
Chlorine reacts with hydrogen in the presence of light and forms hydrochloric acid.
With slaked lime, it makes bleaching powder.
It converts oxides of metals and non-metals into acids.
Moist chlorine can decompose organic matter, such as vegetables, fruits and other edible items.
It reacts with unsaturated hydrocarbons (carbon and hydrogen compounds with double bonds) such as ethene to produce saturated hydrocarbons (carbon and hydrogen compounds with a single bond).
What are the Uses of chlorine?
Chlorine is an essential element that can be used in everyday life. Some of the uses of chlorine are given below:

Chlorine Bleaching Powder
Chlorine acts as a water disinfectant to keep drinking water safe from harmful bacteria.
The element is also useful for household purposes. Chlorine bleach is used to disinfect and whiten clothes. It is also used to clean the bathroom and kitchen surfaces.
Many industries use chlorine as an important substance while manufacturing pesticides. These chlorine-based pesticides help farmers to protect their crops from pests.
The Healthcare industry also uses chlorine. The element is used to manufacture various cholesterol medicines, arthritis pain and allergies.
Chlorine is also used for transportation purposes. It is used to manufacture brake fluid, airbags, bumpers and seat cushions for passengers' safety.
Summary
Chlorine is a naturally occurring gaseous element. The atomic number of chlorine is 17. It demonstrates multiple physical and chemical properties because of its unstable atomic or nuclear structure. This makes it highly reactive, especially towards metals. Moreover, it is used in our daily life activities, such as washing or bleaching clothes, disinfecting water, sanitation, etc. Further, it is used to manufacture pesticides, medicines, electronic items like smartphones, etc. Additionally, it is also used in the transportation industry as it helps to manufacture transport-related items like car seat covers, airbags, brakes and many other devices that ensure the driver’s safety while driving.
FAQs on What Is Chlorine?
1. What are the group and period numbers of chlorine in the periodic table?
Chlorine belongs to group 17 in the periodic table. Its period number is 3. It is a non-metal whose properties are similar to other elements of group 17 like fluorine and bromine. It is the second lightest element in group 17. Chlorine is not found in nature and is available in the form of compounds. The element can be found as solid, liquid or gas. But it is primarily available in the gaseous form. Being an element of group 17, chlorine can combine with other metals to form compounds.
2. What is the structure of chlorine?
The atomic structure of chlorine includes neutrons, protons and electrons. The atomic number of chlorine is 17. This means that the element has 18 neutrons, 17 electrons and 17 protons. The electrons are arranged in three electron shells (electron path). As there are 17 electrons, the first two electrons are arranged in the first electron shell. In the second electron shell, there are 8 electrons. The remaining 7 electrons are arranged in the third electron shell. The protons and neutrons are placed inside the nucleus of the chlorine atom.
3. What is the use of chlorine at home?
Chlorine is used to clean water that comes for domestic purposes. It also protects homes from insects and bugs.





















