




Introduction
In both inorganic and organic chemistry, the word "carbide" is used the most. Now, let's answer the most crucial question: what are carbides? Carbide is a chemical compound of carbon and metal or semimetal elements. It is in the form of ions. The ionic or covalent bond holds the carbide group to the metal or semimetal. The symbol for Carbide is CaC2. It shows that carbide ions have two carbon atoms in them.
Carbide Structure
Structure of Carbide
The carbide structure is a mixture of two carbon atoms joined together by three covalent bonds. There are two pi-bonds among these three covalent bonds. These pi-bonds are made when the p-orbitals touch each other on the side. The sigma bond is another one. It forms when the s-orbitals overlap head-on. The carbon in the carbide structure hybridised in a way called sp. There is one lone pair on each carbon atom.
Use of Carbide
Carbide comes into play in industrial processes and applications. Other kinds serve different purposes. For example, silicon, aluminium, and boron are all elements. They come from various industries.
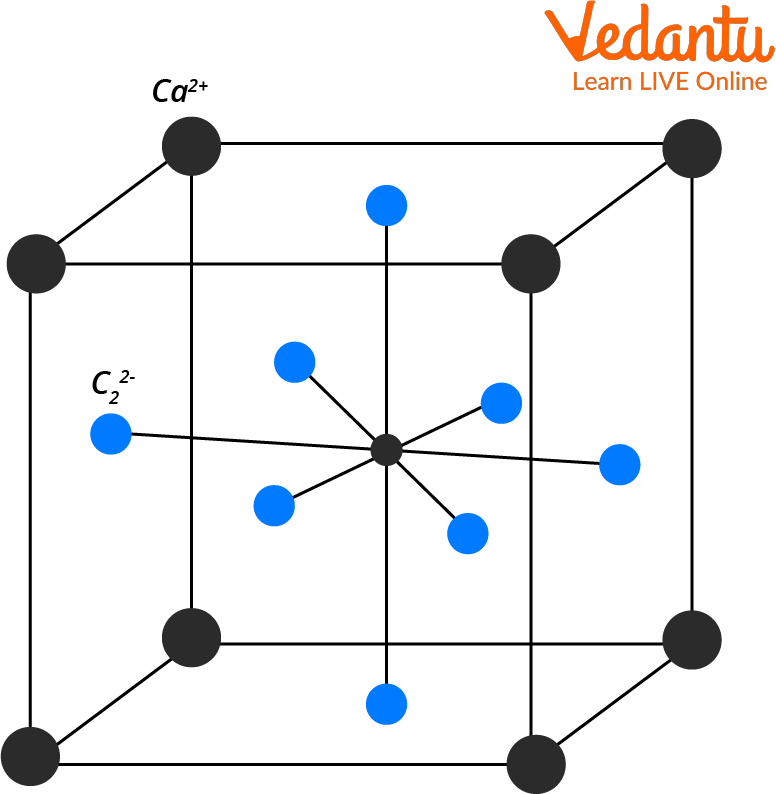
Calcium Carbide
Calcium carbide is a substance that forms in a factory. Its chemical formula is CaC2. When you look at this formula, it's easy to see that one molecule of this forms up of one calcium atom and two carbon atoms.
You will notice that it is clear and solid when it is pure, like rock salt. Most of the time, this chemical compound is used to make acetylene. The calcium carbide uses are as follows below:
The use is to make polyvinyl chloride (PVC) because acetylene, from calcium carbide, can use as a raw material.
They are used to get rid of the sulfur in iron.
Also, as a ripening agent like ethylene, in both big-bang and bamboo cannons.
Silicon Carbide
SiC is the molecular formula for the following chemical compound. So, this molecular formula shows one carbon atom and one silicon atom. It is also known as carborundum.
Silicon Carbide uses in brakes, turbine bearings, and more. It is used in some types of seals and bearings. In the same way, the moissanite jewel, which looks like a diamond, grows large crystals in a lab.
Aluminum Carbide
It comes from making calcium carbide. It looks like it is either yellow or brown. It looks like crystals that can easily dissolve in water. Aluminium carbide uses to prevent damage from happening when added pressure over time.
Magnesium Carbide
With the help of water, magnesium carbide is used to make alkyne or propyne. Alkynes are compounds with two carbon atoms that are linked by three bonds. Alkynes like ethylene and propylene are used for business by adding water to metal carbide. So, magnesium carbide is called allylide, and carbides that can make alkynes are called allylic.
Conclusion
As discussed above, Carbide is a compound form of carbon. The word "carbide" usually means calcium carbide or tungsten carbide. Moreover, carbon carbide is a chemical compound of carbon and metal or semimetal parts. It can be in the form of ions. The number C2-2 stands for Carbide. It means that carbide ions are made up of two atoms of carbon.
FAQs on Let's Learn About Carbide Uses
1. What is a carbide?
A carbide is a chemical compound composed of carbon and a less electronegative element, which is typically a metal or a metalloid. Due to their strong chemical bonds, many carbides are known for their extreme hardness, high melting points, and resistance to heat and wear.
2. What are the main uses of carbides in daily life and industry?
Carbides have a wide range of industrial and commercial uses due to their unique properties. Some key applications include:
- Cutting Tools: Tungsten carbide is used to make drill bits, cutting blades, and other machining tools.
- Abrasives: Silicon carbide is used in sandpaper, grinding wheels, and for polishing hard materials.
- Lighting: Calcium carbide reacts with water to produce acetylene gas, which was historically used in carbide lamps for miners.
- Steel Production: Iron carbide (cementite) is a crucial component in steel and cast iron, giving them strength and hardness.
- Protective Gear: Boron carbide is used in bulletproof vests and tank armour due to its incredible hardness and low density.
3. How is calcium carbide used to produce acetylene gas?
Calcium carbide (CaC₂) reacts with water (H₂O) in a chemical reaction to produce acetylene gas (C₂H₂) and calcium hydroxide. The chemical equation for this process is CaC₂ + 2H₂O → C₂H₂ + Ca(OH)₂. Acetylene is a highly flammable gas that burns with a bright, luminous flame, which is why it was used effectively in carbide lamps.
4. What makes tungsten carbide ideal for cutting tools?
Tungsten carbide (WC) is exceptionally hard, ranking just below diamond on the Mohs scale of hardness. This extreme hardness, combined with its high melting point and resistance to wear, allows it to maintain a sharp cutting edge even at high temperatures and speeds. This makes it perfect for manufacturing high-performance cutting tools, drill bits, and surgical instruments that need to remain sharp.
5. Why are carbides known for their extreme hardness and durability?
The remarkable hardness and durability of many carbides stem from their strong chemical bonding. In carbides like tungsten carbide and silicon carbide, carbon atoms form strong covalent bonds with metal or metalloid atoms. These bonds create a very rigid and stable crystal lattice structure that is extremely difficult to break or deform, resulting in high hardness, wear resistance, and high melting points.
6. How do boron carbide and silicon carbide differ in their applications?
Both are extremely hard ceramic materials, but their primary applications differ based on their specific properties. Boron carbide (B₄C) is exceptionally hard and lightweight, making it ideal for high-performance applications like tank armour, bulletproof vests, and as a control rod in nuclear reactors. Silicon carbide (SiC), while also very hard, is more commonly used as an abrasive in grinding wheels and sandpaper, and as a semiconductor in high-power electronics due to its excellent thermal conductivity.
7. Is it safe to use calcium carbide for artificially ripening fruits?
No, it is not safe and is banned for this purpose in many countries. While the acetylene gas produced from calcium carbide can mimic the natural ripening agent ethylene, industrial-grade calcium carbide often contains toxic impurities like arsenic and phosphorus hydride. These harmful contaminants can remain on the fruit's surface, posing serious health risks to consumers.
8. What are some common examples of carbides besides calcium and tungsten carbide?
Besides the well-known calcium carbide (CaC₂) and tungsten carbide (WC), other important examples include:
- Silicon Carbide (SiC): Also known as carborundum, used as an abrasive and in electronics.
- Iron Carbide (Fe₃C): Also known as cementite, a key component that gives steel its hardness.
- Boron Carbide (B₄C): Used in armour and as a nuclear control material.
- Aluminium Carbide (Al₄C₃): Notable for reacting with water to produce methane gas.









