




Molecules
Molecules are a combination of one or more than one, similar or dissimilar atoms. Everything around us is made up of different combinations of molecules, we ourselves are made up of molecules. The human body mainly consists of water molecules but other than that there are several other types of molecules in our body. The food that we eat is a form of large, complex molecules which are broken down into smaller and simpler molecules in our body, the blood which acts as a conducting part of our body is made up of millions of cells known as the red blood cells which are molecules made up of atoms of iron.
Some Fun Facts About Molecules
Everything around us is made up of molecules.
Our body also is made up of different types of molecules.
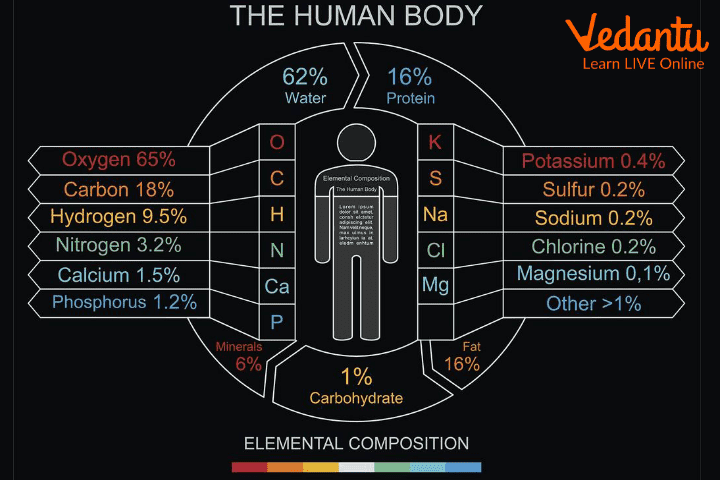
Human Body Made up of Molecules
Molecules can also be of various different shapes and sizes.
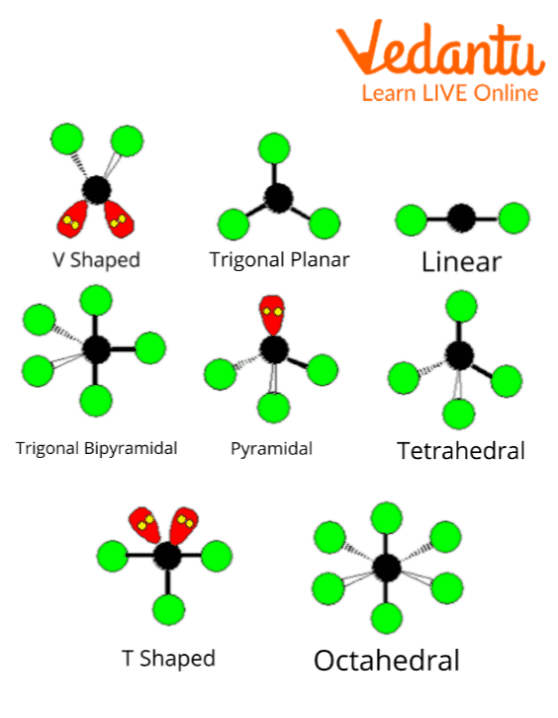
Different Shapes of Molecules
Some molecular formulae for different molecules are:
\[{\rm{C}}{{\rm{H}}_{\rm{4}}}\]- methane
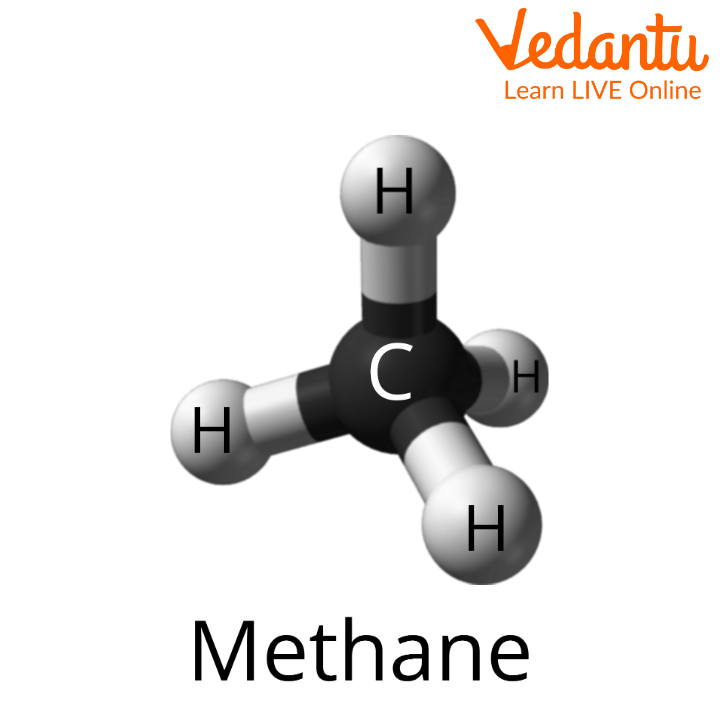
Methane Molecule
\[{{\rm{H}}_{\rm{2}}}{\rm{O}}\] - water
\[{{\rm{H}}_{\rm{2}}}\] - hydrogen
\[{{\rm{O}}_{\rm{2}}}\] - Oxygen

Oxygen Molecule
Complex molecules which are made up of more than one type of atom are termed as a Compound Molecule.
The atoms in a molecule are linked together through chemical bonds, which can be broadly of two types: Ionic Bonds and Covalent bonds.
Ionic Bond is when one atom donates its extra electrons and the other atom accepts the electrons thus forming a bond whereas a Covalent Bond is when there is sharing of electrons between the atoms and no donating or receiving of electrons take place.
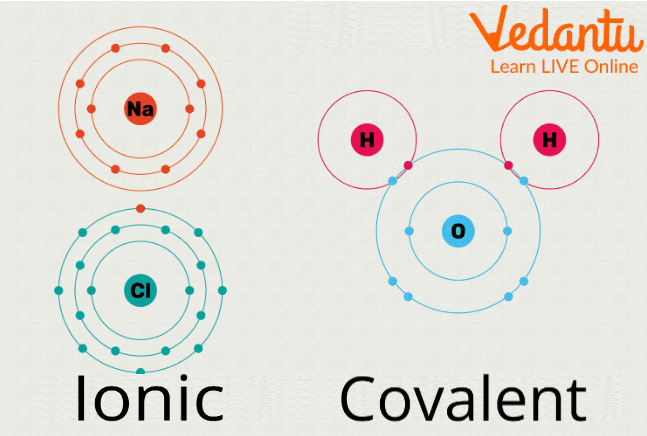
Ionic vs Covalent Bonding Between Atoms
Interesting Facts About Atoms and Molecules
Any time two atoms combine together they result in a molecule.
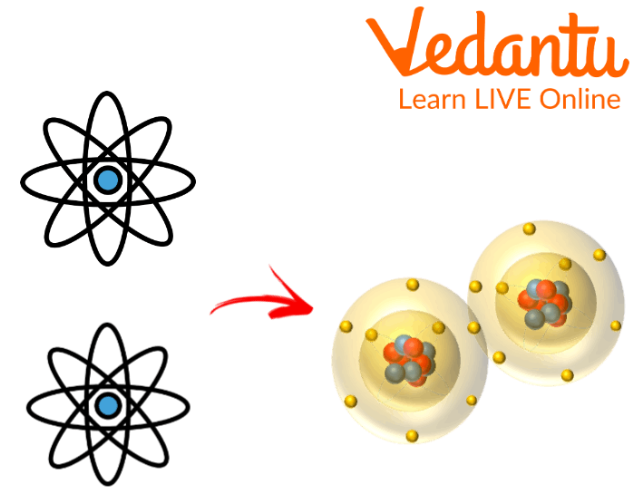
Atoms Forming Molecules
Since molecules are made up of atoms, a molecule can also break down into its component atom; this type of reaction is termed as decomposition reactions.
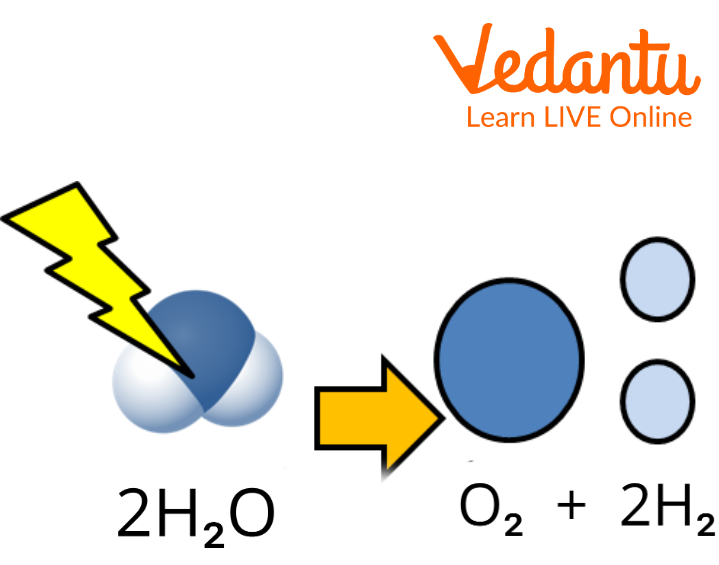
Splitting of Water: A Decomposition Reaction
Initially atoms were thought to be the smallest and indestructible part of nature but later subparticles of an atom were discovered, namely the electrons, protons and neutrons.
The most abundant atom in the universe is Hydrogen atom.
Till date we have discovered about more than 100 different types of atoms, these are listed in something referred to as the periodic table.
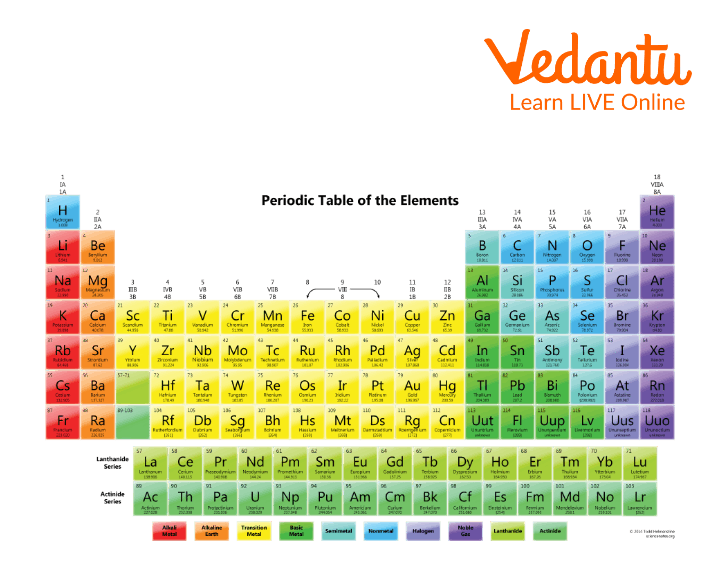
The Periodic Table
Fun Facts about Water Molecules
The water molecule is denoted by \[{{\rm{H}}_{\rm{2}}}{\rm{O}}\] made up of two hydrogen and an oxygen atom.
It is an example of a compound molecule.
Several water molecules are also bonded together by something called Hydrogen Bonding.
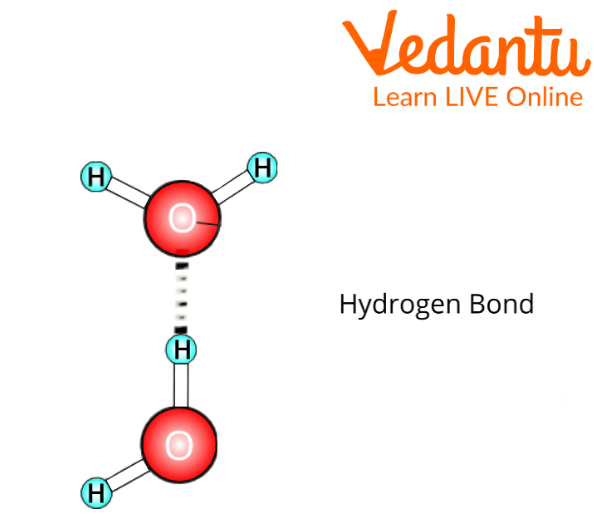
Hydrogen Bonding Between Water Molecules
Water molecules can exist in all three states of matter under normal circumstances.

Water Molecules in Three States of Matter
Usually the solid is more dense than its liquid form and as a result sinks in the liquid, but in this case, ice is less dense than water and thus floats on its surface.

Ice Floating on the Surface of Water.
Sample Questions
State whether the sentences that follow are true or false:
The atom is the ultimate, indestructible substance in nature.
Ans: False, the atom can be subdivided into sub particles like the electrons, protons and neutrons.
Ice is denser than water
Ans: False, ice is not denser than water that is why it floats on water.
Water can exist in all three states of matter.
Ans: True, it can exist in all three states of water as water, ice and vapour.
A molecule can also be made from atoms which are not similar
Ans: True, these types of molecules are known as complex molecules.
2. State the relation between an atom, a molecule and a compound.
Ans: Two or more than two atoms link together to form a molecule, similarly two or more molecules link together to form a compound.
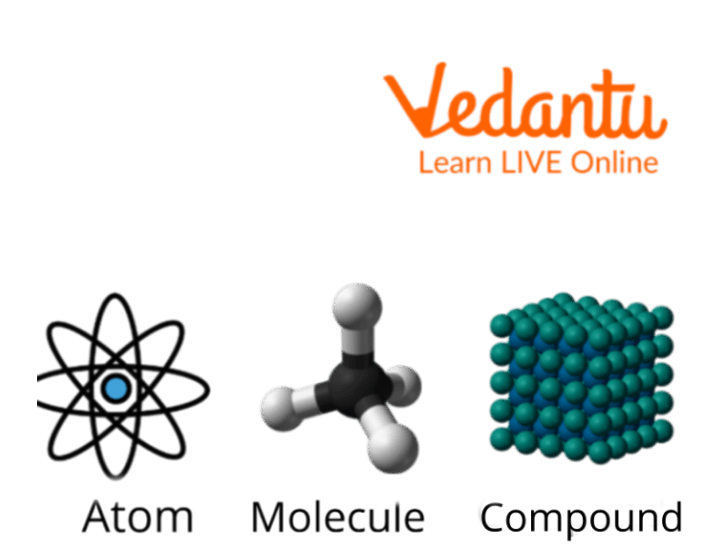
Relation Between Atoms, Molecules and Compounds.
Learn by Doing
Complete the given statements by filling in the blanks with suitable words:
Atoms combine to form a ___________.
Ans: Molecule
Methane is molecularly ________.
Ans: \[{\rm{C}}{{\rm{H}}_{\rm{4}}}\]
Our bodies are made up of _________.
Ans: molecules
Molecules breaking into atoms are referred to as ________.
Ans: Decomposition reaction
Chemical bonds can either be _______ or ________.
Ans: ionic, covalent.
Ice _______ on water.
Ans: floats.
Summary
Two or more than two atoms link together to form a molecule with the help of chemical bonds which can either be ionic or can be covalent in nature. A molecule consequently breaks down into its components that are atoms, and that is termed as a decomposition reaction. A water molecule is a complex molecule made up of two hydrogen and one oxygen atom.
FAQs on Facts About Molecules
1. What is a molecule in simple terms?
A molecule is the smallest particle of an element or a compound that can exist on its own and still retain the chemical properties of that substance. It is formed when two or more atoms are joined together by chemical bonds. For example, a single molecule of water (H₂O) is made of two hydrogen atoms and one oxygen atom.
2. What are molecules made of?
Molecules are made of atoms. Depending on the substance, a molecule can be formed from:
- Atoms of the same element: For example, an oxygen molecule (O₂) that we breathe is made of two oxygen atoms joined together.
- Atoms of different elements: For example, a carbon dioxide molecule (CO₂) is made of one carbon atom and two oxygen atoms.
3. What force holds the atoms together inside a molecule?
The atoms inside a molecule are held together by strong attractive forces called chemical bonds. These bonds are created when atoms share or exchange electrons, which allows them to form a stable unit. This bonding is what gives a molecule its unique structure and properties.
4. What are some examples of molecules found in everyday life?
We are surrounded by molecules. Here are a few common examples:
- Water (H₂O): The molecule essential for all known life.
- Oxygen (O₂): The gas in the air that we need to survive.
- Methane (CH₄): The main component of natural gas used for cooking and heating.
- Sugar (C₁₂H₂₂O₁₁): The sucrose molecule that we use to sweeten food.
- Vinegar (CH₃COOH): The acetic acid molecule that gives vinegar its sour taste.
5. How is a molecule of an element different from a molecule of a compound?
The main difference is in the type of atoms they contain. A molecule of an element is made up of atoms of only one kind, such as in Nitrogen (N₂) or Ozone (O₃). In contrast, a molecule of a compound is made up of atoms from two or more different elements combined in a fixed ratio, like in water (H₂O) or ammonia (NH₃).
6. Can a single atom also be considered a molecule?
Yes, in certain cases. The molecules of noble gases, such as Helium (He) and Argon (Ar), are composed of just a single atom. This is because these elements are extremely stable and do not need to bond with other atoms. Such molecules are described as being monoatomic.
7. Why is the shape of a molecule important?
A molecule's three-dimensional shape is crucial because it determines many of its physical and chemical properties, including its smell, taste, and how it interacts with other molecules. In biology, for example, the specific shape of an enzyme molecule allows it to function correctly by binding only to certain substances, much like a key fitting into a lock.
8. Are molecules always moving?
Yes, molecules are in a constant state of motion. The extent of this motion depends on the state of matter:
- In gases, molecules move around rapidly and randomly.
- In liquids, molecules can slide past one another.
- In solids, molecules are locked in place but still vibrate continuously.









