




Why Carbon Is Essential: Unique Bonding, Allotropes & Applications
All organic life on earth is carbon-based. The quantity or the amount of carbon in nature is quite small, yet it forms the most complex array of compounds from diamonds to DNA.
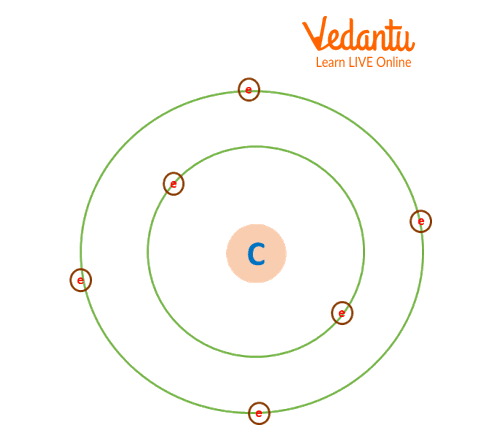
The uniqueness of carbon among all the elements owes to its electronic configuration. It has the atomic number 6. Two electrons fill the inner orbit and four electrons occupy the outer orbit. It requires four electrons to fill its octet, giving it a valency of four.
The unique properties of carbon that makes it the most versatile element on earth can be attributed to its:
Tetravalency
Catenation
Tetravalent Carbon
Carbon Bonding
Given its small size, the hold of the carbon nuclei on the electron helps it form strong stable bonds with other elements.
As mentioned earlier, carbon needs four electrons to fulfil its octet, accepting four electrons would lead to four negative charges on the atom which would make it a very unstable system. Carbon extensively forms covalent bonds with other elements to satisfy its octet requirement.
In a covalent bond, two atoms would come together to share their electron and an effective sharing at an optimum distance will form a bond. This property allows carbon to form four bonds with four monovalent elements at one time. The size helps it hold on to the shared pair of electrons and it goes on to form covalent bonds with a wide range of elements such as hydrogen, oxygen, nitrogen, sulphur, chlorine etc.
Catenation
Carbon is the only atom that can form long chains with numerous architectures. The self-linking property of carbon atoms is known as catenation. One carbon can at most form a bond with four other carbons, each of which can propagate the chain by linking to other carbon while satisfying its tetravalency. This rudimentary binding format creates the hardest compound on earth - diamond.
The catenating ability of carbon is successful primarily due to its small size, which avoids severe steric clashes between the atoms. Catenation is also observed in silicon but is not as extensive as in carbon, because its size is bigger than carbon and it has a more diffused electron cloud, which causes electronic repulsion with adjacent atoms.
Carbon forms long chains by linking with each other, the chains can be linear, but there can also be branches. Carbon atoms can also be arranged in cyclic rings. Apart from single bonds, carbon can also form double and triple bonds. The multiplicity of bonds helps carbon form compounds with other elements like nitrogen and oxygen.
Carbon compounds have another interesting feature of isomerism. Isomers are compounds that have the same molecular formula but different structures, which means the same number of atoms can be arranged in multiple ways to have a different structure with different properties.
This gives carbon compounds tremendous versatility.
Some Unique Classes of Carbon Compounds
Carbon compounds with at least one metal bond are called organometallic compounds. Examples: Ferrocene, Zeise's Salt, and tetraethyl lead.
Carbon forms a binary compound with another lower electronegativity element called carbides. Examples: CaC2 , Al4C3 , SiC , TiC.
Carbon-containing bonded halogens are called Carbon Halides. Example: CCl4 (carbon tetrachloride), CI4 (carbon tetraiodide).
Molecular clusters made up of carbon and boron atoms form a unique class of compounds called Carboranes. Example H2C2B10H10.
Uses
Carbon is used in the form of petrochemical products.
Carbon is used to form various commercial polymers, such as plastics, which are synthetic carbon polymers.
Carbon steel which is a carbon alloy with iron is used to produce high-strength wires and machines.
Carbon allotrope graphite mixed with clay is used to form lead that is used to make pencils.
Carbon allotrope diamond is used as a gem, and in industry, it is utilised in cutting and polishing tools.
Carbon black is used in the printing ink, carbon papers, and in rubber products.
Activated Charcoal is used in filters as an absorbent and adsorbent material, which can filter toxins.
Interesting Facts
More than one million compounds of carbon are known so far.
Carbon has a large number of allotropes, the most familiar of them are diamond and graphite. New allotropes of allotropes carbon such as fullerene (C60) and Graphenes have been discovered in recent decades.
Key Features
Carbon is the basis of all life.
Carbon has an atomic number of 6.
Carbon has a valency of four (Tetravalency).
Carbon can link with other carbon atoms to form large chains. This is called catenation.
Carbon can form multiple bonds (double and triple).
FAQs on Versatile Nature of Carbon: Concepts, Examples & Importance
1. What is meant by the versatile nature of carbon?
The versatile nature of carbon refers to its unique ability to form a vast number of stable compounds with a wide variety of structures and properties. This versatility is the foundation of organic chemistry and is primarily due to two key properties: catenation and tetravalency. These allow carbon to form long chains, branched chains, and ring structures, creating an almost limitless number of molecules.
2. What are the two main properties of carbon that lead to its versatile nature?
The two primary properties responsible for carbon's versatile nature as per the CBSE Class 10 syllabus are:
- Catenation: This is the self-linking property of carbon atoms to form long, stable chains and rings through strong covalent bonds.
- Tetravalency: Carbon has a valency of four, meaning each carbon atom can form four covalent bonds with other atoms, allowing for diverse and complex three-dimensional molecular structures.
3. How does catenation contribute to the vast number of carbon compounds?
Catenation is carbon's exceptional ability to form strong covalent bonds with other carbon atoms. This property allows for the creation of diverse carbon skeletons, such as:
- Long straight chains of carbon atoms (e.g., in butane).
- Branched chains where carbon chains have other chains attached (e.g., in isobutane).
- Ring structures where carbon atoms form closed loops (e.g., in cyclohexane).
4. What is tetravalency, and how does it explain carbon's ability to form stable compounds?
Tetravalency means that a carbon atom has four valence electrons and can therefore form four covalent bonds. This allows carbon to bond with four other atoms, which can be other carbon atoms or atoms of different elements like hydrogen, oxygen, nitrogen, and halogens. Due to its small atomic size, carbon's nucleus holds the shared electron pairs very strongly, resulting in stable covalent bonds and the formation of robust molecules essential for life and industry.
5. What are some common examples of carbon's allotropes?
Allotropes are different structural forms of the same element. Carbon has several important allotropes, each with unique properties. The most common examples are:
- Diamond: A very hard, transparent crystal where each carbon atom is bonded to four others in a rigid tetrahedral lattice.
- Graphite: A soft, greyish-black solid where carbon atoms are arranged in hexagonal layers that can slide over each other, making it a good lubricant.
- Fullerenes: Molecules composed entirely of carbon, forming a hollow sphere or tube, with Buckminsterfullerene (C60) being a well-known example.
6. Besides catenation and tetravalency, what other factor contributes to carbon's versatility?
Another crucial factor is carbon's ability to form multiple bonds (i.e., double and triple covalent bonds) with itself and other elements like oxygen and nitrogen. The formation of single (C-C), double (C=C), and triple (C≡C) bonds allows for the creation of saturated and unsaturated compounds with distinct chemical properties, such as alkanes, alkenes, and alkynes, further expanding the diversity of organic molecules.
7. If silicon also has tetravalency, why is it not as versatile as carbon?
Although silicon is in the same group as carbon and also has a valency of four, it is not as versatile because its atomic size is larger. This larger size results in weaker Si-Si bonds compared to the strong C-C bonds. Consequently, long chains of silicon atoms (silanes) are much less stable and more reactive than carbon chains. Furthermore, silicon does not readily form stable double or triple bonds, which severely limits the variety of compounds it can form compared to carbon.
8. What are some real-world examples that demonstrate the importance of carbon's versatility?
Carbon's versatility is fundamental to our world. Its importance is seen in:
- Life Itself: All major biological molecules, including DNA, proteins, carbohydrates, and fats, are built on a carbon backbone.
- Fuels: Fossil fuels like petrol, diesel, and natural gas are mixtures of hydrocarbons, which store large amounts of energy in their C-C and C-H bonds.
- Modern Materials: Everyday materials like plastics, polymers, synthetic fibres (nylon), and pharmaceuticals are all complex organic compounds designed around specific carbon frameworks.
























