




Key Features and Examples of Seesaw Molecular Geometry
There are three steps that help in determining the shapes of molecules. The first is to know the Lewis dot structure of the compound which helps in to identify the bond pairs and the lone pairs. The second is to determine the electron geometry from the Lewis dot structure and lastly to determine the molecular geometry. The valence-shell electron-pair repulsion (VSEPR) theory is used to determine the molecular geometry and the electron-group geometry.
Molecular geometry helps in understanding the shape of the molecule by the three-dimensional arrangement of the atoms that make up a molecule. It contains all the geometric parameters which determine the overall shape of the molecule, as well as bond lengths, bond angles, torsional angles, and any other geometrical properties that govern the position of each atom.
What is Seesaw Molecular Geometry?
There are many molecular geometries based on the arrangement of atoms. Seesaw is one of the molecular geometries of an atom. In this type of molecular geometry, the central atom has four bonding groups and one lone pair of electrons on it. This shape got its name because the Bond is observed in the shape of a playground seesaw.
Seesaw Shape
Seesaw-shaped molecules are much rarer than trigonal bi-pyramidal and tetrahedral molecules. A trigonal pyramid is formed when a central atom is attached to five different atoms out of which two are bonded axially and three equatorially with no lone pair. However, in the seesaw model, the central atom is surrounded by four adjacent atoms, two of which are on the same plane (axial) and two of which are below (equatorial) with a lone pair. A single pair of electrons on the central atom causes this shape.
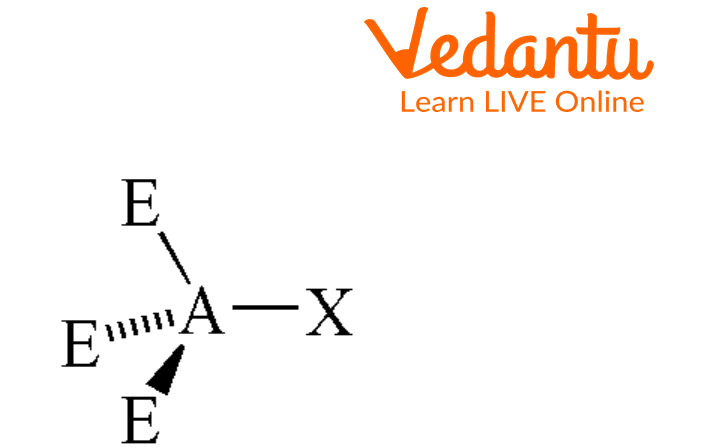
Tetrahedral Geometry
The above image shows the tetrahedral geometry of the molecule which has a bond angle of 109.5 degrees. Four atoms are bonded with the central atom by sigma bonds.
Seesaw Molecular Geometry Bond Angles
The seesaw shape has 5 electron density regions (trigonal bipyramidal), with four bonding pairs and one lone pair. When all of these regions are bonding, the molecule has a 120-degree in trigonal shape and 90-degree angles between the two atoms that make up the "bipyramidal" part of the shape.
In Seesaw molecular geometry where there are four bonds attached to a central atom and one lone pair. When a lone pair is added, it is placed as far away as possible from the bonding pairs due to electron-electron repulsion. A molecule with seesaw molecular geometry has a bond angle of less than 90° and 120°.
Seesaw Molecular Geometry Hybridization
Seesaw appearance in molecular geometry is due to a lone pair of electrons on the central atom. Tellurium tetrachloride, or TeCl4, is an example of a seesaw-shaped molecule. The core element is tellurium, with four chlorine atoms attached to it.
The form of TeCl4 is see-saw-like. Te is sp3d hybridized in the molecule TeCl4 and has four coordination numbers due to its four bonds with chlorine. Because it resembles a seesaw, TeCl4 is considered to be in a seesaw shape. Hence, TeCl4 shows seesaw molecular geometry.
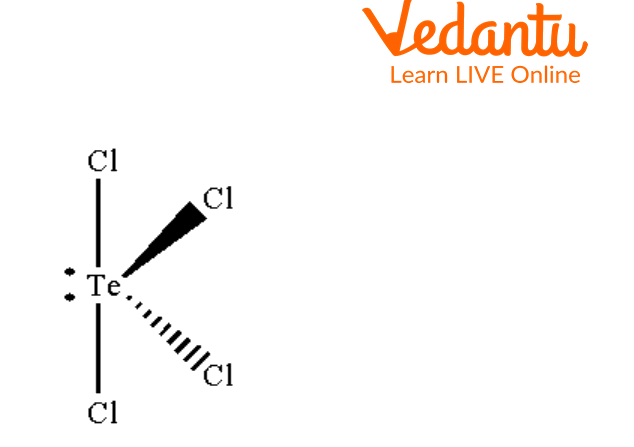
See-Saw Structure of TeCl4
The above image shows the see-saw structure of TeCl4. Te is bonded with four chlorine atoms by sigma bonds and one lone pair of electrons is present on Te atom.
Examples of Seesaw Molecules
Some examples of seesaw molecules are Sulphur tetrafluoride (SF4), Selenium tetrafluoride (SeF4), Tellurium tetrafluoride (TeF4), Xenon Dioxide Difluoride (XeO2F2), Sulphur tetrachloride (SCl4), and Tetrafluoroarsanuide (AsF4). In all of these examples, a central atom has four different bonds surrounding and one lone pair which results in the seesaw shape of the molecule. Bond angles in seesaw shape are less than 180 degrees at axial bonds and less than 90 and 120 degrees at an equatorial angle.
The structure of SF4 is given below: The Central S atom has sp3d hybridization. In the below image of the SF4 structure, the central S atom has four fluorine atoms attached to it with one lone pair giving it a seesaw shape.
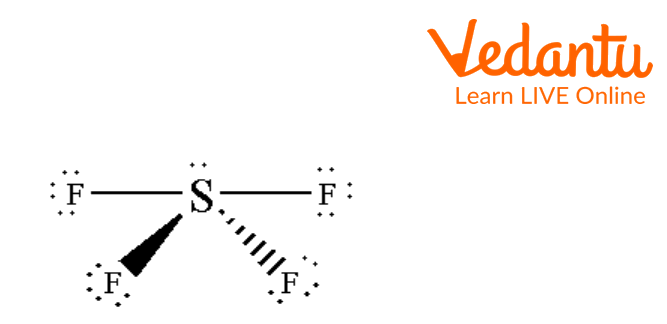
See-saw Structure of SF4
The above image is the see-saw shape of SF4. The sulphur atom is bonded with four fluorine atoms by sigma bonds and one lone pair is present on the Sulphur atom.
Another example of a seesaw shape is Xenon Dioxide Difluoride. Below is given a structure of a Xenon Dioxide Difluoride where Xe has four atoms attached to it with one lone pair giving it a seesaw shape. Central Xe has sp3d hybridization.
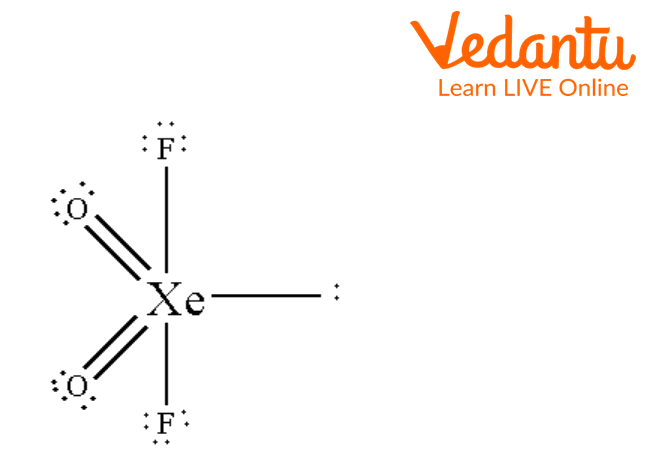
See-saw Structure of XeO2F2
The above image is the see-saw structure of XeO2F2. The Xe atom is bonded with two oxygen atoms by a double bond and with two fluorine atoms by single bonds. One lone pair is present on Xe.
Key Features
Seesaw molecular geometry has one lone pair and four bonds surrounding the central atom.
One lone pair is responsible for the seesaw shape of molecules which got its name from its resemblance to the seesaw in the playground.
The seesaw shape forms a bond angle of less than 90 to 120 degrees.
Seesaw molecular geometry is polar. Some examples of a seesaw shape are formed by molecules such as SF4, TeCl4, AsF4, etc.
FAQs on Seesaw Molecular Geometry Explained for Students
1. What is meant by seesaw molecular geometry?
Seesaw molecular geometry, also known as disphenoidal geometry, describes the shape of a molecule where a central atom is bonded to four other atoms and has one lone pair of electrons (an AX₄E₁ type). It is derived from a trigonal bipyramidal electron geometry. The name comes from its resemblance to a playground seesaw.
2. What type of hybridisation leads to a seesaw molecular geometry?
Molecules exhibiting seesaw geometry have a central atom with sp³d hybridisation. This hybridisation involves one s, three p, and one d orbital, creating five electron domains. When these domains are arranged as four bonding pairs and one lone pair, the resulting molecular shape is a seesaw.
3. What are the ideal bond angles in a molecule with seesaw geometry?
In a seesaw geometry, the bond angles deviate from the ideal trigonal bipyramidal angles due to repulsion from the lone pair. There are three types of angles:
- The angle between the two axial atoms is less than 180°.
- The angle between the two equatorial atoms is less than 120°.
- The angle between an axial and an equatorial atom is less than 90°.
4. What are some common examples of molecules with a seesaw geometry?
Some classic examples of molecules that adopt a seesaw shape, as studied in the CBSE Class 11 syllabus, include:
- Sulphur tetrafluoride (SF₄)
- Selenium tetrafluoride (SeF₄)
- Tellurium tetrachloride (TeCl₄)
- Iodine tetrafluoride cation (IF₄⁺)
5. How is the seesaw geometry related to the trigonal bipyramidal shape?
The seesaw shape is a direct derivative of the trigonal bipyramidal electron geometry. A trigonal bipyramidal arrangement has five electron domains. If one of these domains is a non-bonding lone pair of electrons (specifically in an equatorial position to minimise repulsion), the remaining four bonding pairs arrange themselves into a shape that we identify as a seesaw.
6. Why does the lone pair in a seesaw geometry occupy an equatorial position instead of an axial one?
According to VSEPR theory, electron pairs arrange themselves to minimise repulsion. A lone pair in an equatorial position experiences repulsion from only two atoms at ~90°. If it were in an axial position, it would face stronger repulsion from three atoms at 90°. Therefore, the equatorial position is more stable and energetically favourable, leading to the seesaw shape.
7. How does the seesaw geometry differ from a tetrahedral shape?
The key difference lies in the presence of a lone pair on the central atom.
- Seesaw Geometry (AX₄E₁): Has a central atom with four bonding pairs and one lone pair. This results in an asymmetrical shape with multiple, non-ideal bond angles.
- Tetrahedral Geometry (AX₄): Has a central atom with four bonding pairs and zero lone pairs. It is a highly symmetrical shape with uniform bond angles of 109.5°.
8. Why does Sulphur Tetrafluoride (SF₄) have a seesaw shape?
In SF₄, the central sulphur atom has six valence electrons. It forms four single bonds with four fluorine atoms and has one remaining lone pair. This gives a total of five electron domains (4 bonding + 1 lone). As per VSEPR theory, five electron domains arrange in a trigonal bipyramidal electron geometry. With one position occupied by a lone pair, the resulting molecular shape is a seesaw.
























