




Why Is Mendeleev’s Periodic Table Important in Chemistry?
The chemical elements are shown in tabular form on the periodic table, commonly referred to as the periodic table of the (chemical) constituents. It is frequently employed in physics, chemistry, and related sciences and is regarded as a sign of chemistry. There are 118 elements listed in Mendeleev’s Periodic Table.
Out of them, 94 elements comprise indigenous elements, and 24 are made of synthetic materials. There were just 30 discovered elements in the year 1800. Scientists found it difficult to memorise the elements and their attributes as an increasing number of elements were discovered. They began compiling and classifying data related to the elements. It became common practice to group items into tabular categories based on their characteristics.
A periodic table is a tabular form that groups different elements into groups based on how they behave. Mendeleev's periodic table is a visual representation of the Mendeleev periodic law, which claims that the atomic numbers of chemical elements have a periodic influence on those elements' attributes. Thus, this article provides more detailed information on the table and its merits and demerits.
Define Mendeleev Periodic Law
According to Mendeleev's periodic law, an element's atomic weight is a periodical function of both its physical and chemical characteristics. Mendeleev postulated that the periodical physical and chemical characteristics of elements are based on their atomic weight in such a way that when they are arranged in a rising sequence of atomic weight, elements with comparable characteristics are replicated at periodic gaps of expanding atomic weight.
Mendeleev’s Periodic Table
Russian scientist Dmitri Ivanovich Mendeléev made the most significant contribution to the initial creation of the periodic table. Mendeleev's periodic table was the finest fundamental of several periodic tables that were created.
Once the Newlands Octave Law was rejected in 1869, Mendeleev's Periodic Table entered the scene. In Mendeleev's periodic table, elements were grouped according to their basic characteristic, atomic mass, and molecular characteristics. Just 63 elements were discovered at the time of Mendeleev's research. Mendeleev discovered a periodic relationship between the characteristics of elements and atomic mass after researching the characteristics of each element.
He organised the elements so that they fell within the similar vertical columns of the periodic table and had comparable qualities. These vertical columns were known as Groups by Mendeleev, while the next horizontal rows were known as Series. The accomplishment of Mendeleev can be attributed to his idea of classifying elements depending on the resemblance in their empirical formulas and the characteristics of the compounds they can produce. This caused him to sometimes deviate from the order in which atomic weights group alongside elements with comparable characteristics.
For instance, due to the similarity in their characteristics, fluorine, chlorine, and iodine, all of which have lower atomic weights than tellurium, were all assigned to Group 7 together with iodine.
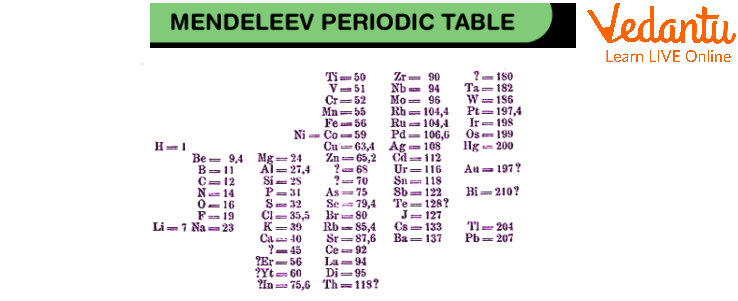
Mendeleev Periodic Table
Mendeleev‘s Modern Periodic Table
The prolonged format of the periodic table is the current version that is commonly utilised around the world. The vertical columns and horizontal rows in this type of periodic table are referred to as the groups and the periods, respectively.
Element groupings are made up of atoms with comparable outer shell electronic configurations. The groups' former names were IA,...VIIIA, VIII, IB,...VIIB, and 0. However, today they go by the numbers 1, 2, 3...18. Periods are represented by the 7 horizontal rows in the current periodic table. The period of the element is determined by the fundamental quantum number n.
Among the 4 quantum numbers (n, l, m, and s), one is the principal quantum number (n). It provides information about the fundamental electron shell. For instance, if n=3, it means that the primary shell is number 3.
Merits of Mendeleev’s Periodic Table
Mendeleev's periodic table had gaps since not all elements were discovered at the time; so, if a novel element is found, it can be added to a new group without affecting the already existing groups.
He was also able to forecast certain of the unknown elements' properties using the periodic law.
Demerits of Mendeleev’s Periodic Table
Mendeleev searched the periodic table for hydrogen but couldn't locate it.
While migrating from one element to the next, the rise in atomic mass was inconsistent. Therefore, it was unpredictable how many elements were still to be identified.
Eventually, isotopes of some elements were discovered that went against Mendeleev's periodic law.
Interesting Facts
The Mendeleev periodic law, regarded as a significant finding in the late 19th century, was described by the finding of the atomic number and ground-breaking research in quantum mechanics in the early 20th century, which revealed the underlying atomic structure.
The characteristics of an unidentified element that must fit beneath aluminium in the table were predicted by Mendeleev.
The properties of that element, known as gallium, were determined to be rather closer to Mendeleev's forecasts when it was identified in 1875. Mendeleev's periodic table was further validated by the eventual discovery of two additional anticipated elements.
Key Features to Remember
In 1869, Mendeleev introduced his periodic table. His organisation of chemical elements was focused on atomic mass.
Mendeleev's periodic table made it feasible to anticipate characteristics of elements which had yet to be identified.
The vertical columns were known as Groups by Mendeleev, while the next horizontal rows were known as Series.
Every version of Principles of Chemistry was revised by Mendeleev, who included all new scientific information, especially evidence for the periodic law, and re-analysed any obstacles to its validation (radioactivity, and rare-earth, inert gases elements).
FAQs on Mendeleev’s Periodic Table: Concepts, Properties & Uses
1. What is Mendeleev's Periodic Law?
Mendeleev's Periodic Law states that the physical and chemical properties of elements are a periodic function of their atomic masses. This means that when elements are arranged in order of increasing atomic mass, elements with similar properties recur at regular intervals.
2. What were the main characteristics of Mendeleev's periodic table?
Mendeleev's periodic table was unique for its time due to several key characteristics:
Basis of Classification: It was primarily based on arranging elements in order of increasing atomic mass.
Systematic Arrangement: Elements were organised into vertical columns called groups and horizontal rows called periods.
Grouping by Properties: Elements within the same group exhibited similar chemical properties, particularly in the formulae of their hydrides and oxides.
Gaps for Undiscovered Elements: Mendeleev intentionally left gaps for elements that he predicted were yet to be discovered.
3. What is the significance of the 'eka-elements' in Mendeleev's table?
The 'eka-elements' represent one of the most significant achievements of Mendeleev's periodic table. He left gaps for undiscovered elements and predicted their properties with remarkable accuracy by studying the trends of their neighbouring elements. For example:
Eka-aluminium was his prediction for an element below aluminium, which was later discovered as Gallium (Ga).
Eka-silicon was his prediction for an element below silicon, later discovered as Germanium (Ge).
The eventual discovery of these elements with properties closely matching his predictions provided strong validation for his periodic law.
4. What were the major limitations of Mendeleev's periodic table?
Despite its success, Mendeleev's periodic table had several significant limitations:
Position of Hydrogen: Hydrogen's position was not fixed, as it resembled both the alkali metals (Group I) and the halogens (Group VII).
Position of Isotopes: Since isotopes of an element have different atomic masses but the same chemical properties, they would require different positions in his table, which would disrupt the periodic arrangement.
Anomalous Pairs: At certain places, an element with a higher atomic mass was placed before one with a lower atomic mass (e.g., Tellurium before Iodine) to maintain the similarity in properties.
5. How did Mendeleev's periodic table help in correcting the atomic masses of some elements?
Mendeleev's periodic table played a crucial role in correcting the doubtful atomic masses of several elements by placing them in their correct positions based on their properties. For instance, the atomic mass of Beryllium (Be) was initially thought to be 13.5. However, based on its position in the table, Mendeleev suggested its valency should be 2, not 3, which led to the correction of its atomic mass to approximately 9. This new value was consistent with its placement above Magnesium.
6. Why was the position of hydrogen ambiguous in Mendeleev's classification?
The position of hydrogen was a major anomaly because it exhibited dual behaviour, showing similarities with two different groups:
Resemblance with Alkali Metals (Group I): Like alkali metals, hydrogen forms a positive ion (H+), has a valency of 1, and combines with halogens, oxygen, and sulphur to form similar compounds (e.g., HCl, H₂O, H₂S).
Resemblance with Halogens (Group VII): Like halogens, hydrogen exists as a diatomic molecule (H₂) and can form a negative ion (H⁻), known as a hydride ion. It also forms covalent compounds with metals and non-metals.
Because of this dual nature, a definitive, single position for hydrogen could not be justified in Mendeleev's table.
7. How does Mendeleev's periodic table fundamentally differ from the Modern Periodic Table?
The primary difference lies in the fundamental property used for classification. Mendeleev's table was based on atomic mass, while the Modern Periodic Table, proposed by Henry Moseley, is based on atomic number. This change resolved the anomalies of Mendeleev's table, such as the position of isotopes (which now occupy the same position) and the order of anomalous pairs like Tellurium and Iodine.
8. Which chemical element is named in honour of Dmitri Mendeleev?
The chemical element named in honor of Dmitri Mendeleev is Mendelevium (Md). It is a synthetic radioactive element with the atomic number 101. It was named to recognize his pioneering and fundamental contributions to the systematic classification of elements and the formulation of the periodic law.
























