




An Overview of Class 11 Physics To Study The Variation In Volume With Pressure For A Sample Of Air At Constant Temperature Experiment
In daily life, we can see the application of Boyle's law. One of the important uses of Boyle's law is to fill the bike tire with air. The gas molecules inside the tire are squeezed and packed closer together as air is pumped into it. It increases the force applied to the tire's walls. Also, the air in balloons is filled by the use of Boyle’s law.
By pumping air into the balloon, the balloon is enlarged, and the balloon expands as a result of the air's pressure pulling on the rubber. When one end of the balloon is compressed, the pressure inside the balloon increases, which causes the uncompressed portion of the balloon to expand outward.
Table of Content
Aim
Properties of gases and gas laws
Result
Aim
To study the variation in volume with pressure for a sample of air at constant temperature by plotting graphs between P and V and between P and 1/V.
Apparatus Required
Boyle’s law apparatus
Plumb line
A pair of set-squares
A thermometer
Fortin’s barometer
Theory
Properties of Gases and Gas Laws
A substance that lacks a defined shape or volume is said to be in a gaseous condition. It moulds and takes the dimensions and shape of its container. Pressure, volume, temperature, and gas mass are the basic macroscopic characteristics of gases. They can be explained by kinetic theory by taking into account both the structure and motion of their molecules. According to scientific observation, these variables are related to one another, and the values of these properties determine the condition of the gas.
These relations between a gas's pressure, temperature, and volume result in gas laws. According to Boyle's Law, gas volume increases as pressure lowers. Charles' Law states that the volume of gas rises with temperature, and Avogadro's Law states that the volume of gas rises with gas concentration. The ideal gas law is formed by combining the three simple gas laws.
Boyle's law
According to Boyle's law, a gas's temperature (T) remains constant while its pressure (P) is inversely proportional to its volume (V).
For an enclosed tube, at a constant temperature (T):
P ∝ 1/V
PV = constant
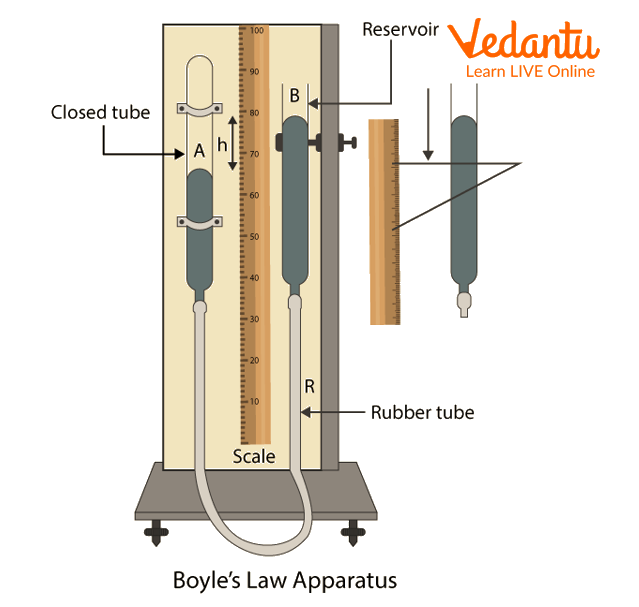
Boyle’s Law Apparatus
In this experiment, Boyle's law apparatus comprises two glass tubes that are each about 25 cm long and 0.5 cm in diameter. One end of tube AB is closed, and the other end of tube CD is open. At the opposite end, a small hole is drawn into the two tubes (B and D). A rubber tube with a thick wall joins the ends B and D. Along the metre scale, the glass tube AB is fastened vertically. With the aid of screw S, the other tube CD may be positioned vertically along a vertical rod and fixed to it at any height.
The rubber tubing, CD tube, and AB tube are all filled with mercury. There is some air trapped in the closed tube AB. Since the air column has a uniform cross-section, its length and volume are proportional. The device is fixed on a platform that is horizontal with a vertical stand. There are levelling screws.
Procedure
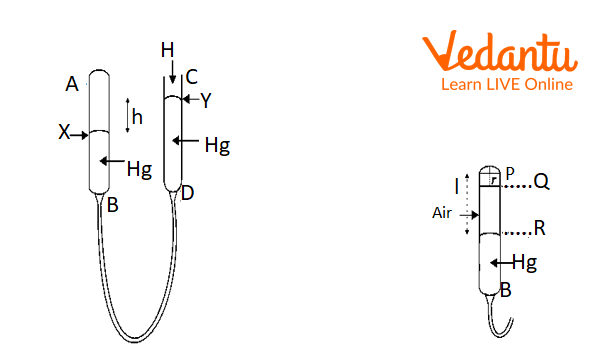
Pressure of Air in Tube AB and Volume of Air Trapped in Tube AB.
Measurement of Pressure
The difference (h) between the mercury levels (X and Y) in the two tubes AB and CD are used to calculate the pressure of the contained air in tube AB. Since liquid in connected vessels has the same pressure at any horizontal level.
Therefore, P( Pressure of enclosed air) = H ± h
Where ‘H’ is the atmospheric pressure.
Measurement of volume of trapped air
If the closed tube is not graduated, then the volume of air in the tube will be = Volume of air in the portion PR - Volume of air in curved portion PQ
Considering that r is the tube's radius,
The volume of the curved portion = volume of the hemisphere having radius r = 1/ 2 × 4/3 πr³ = 2/ 3 πr³
Volume of curved portion PQ = πr² x r = πr³
Therefore, error in the volume= πr³- 2/ 3 πr³ = 1/ 3 πr³
So, the resulting error in the length= (1/ 3 πr³) / πr²
The correction in length = - 1/ 3 r = 1/ 3 PQ ……………………………… eq(i)
To measure the accurate length value in eq(i) should be subtracted from the
measured length l.
We know that according to Boyle’s Law,
P ∝ 1/V
PV = constant,
Therefore, the graph between P and V is a curve line and the graph between P-
1/V is a straight line.
Measurement of the volume of air for a given pressure.
Using a thermometer, record the room's temperature.
Use Fortin's Barometer to measure the air pressure.
Using the levelling screws and spirit level, position the device vertically.
Move the tube CD such that the mercury level is the same as in AB. To read the upper convex meniscus of mercury, use the set square.
Take note of the reading on the metre scale that corresponds to the closed tube P's top end and level Q's reading at the point where its curvature just ends. Determine 1/3 PQ and record it.
To make the mercury levels in tubes AB and CD different, raise CD. Read the mercury meniscus X and Y in tubes AB and CD carefully using the set square. Take note of the mercury level difference of h.
Repeat the CD adjustment for a further five 'h' values. This needs to be executed smoothly and slowly. Boyle's law won't hold true if CD is moved away from AB quickly; doing so guarantees that there isn't a change in temperature.
Determine the closed tube's diameter using the vernier callipers, and from there, calculate the radius, r, which is equal to 1/ 3 of PQ.
Record your observations in the observation table.
Plot (i) the P versus V graph and (ii) P versus 1/ V, and interpret the graphs.
Observation
Note the room temperature = ... °C.
The atmospheric pressure when observed from the Fortin's Barometer = ... cm of Hg.
For the correction in level l because of the curved portion of tube AB
(a) Note for the top of the closed tube AB (P) = ... cm.
Note where the uniform portion of the tube AB begins (or the curved portion ends) (Q) = ... cm.
The (error) Difference between (P – Q) = r = ... cm.
The correction in level l = 1/ 3 r = …
OR
(b) Diameter of the tube AB = d = ... cm.
Therefore, the radius r = ½ d = ... cm.
And the correction for level l = ⅓ r
Measurement of Pressure and Volume of Enclosed Air
Result
We concluded that the graph between P and V is a curve within experimental limits.
And the product PV is a constant. Thus, the graph between P - 1/V is a straight line.
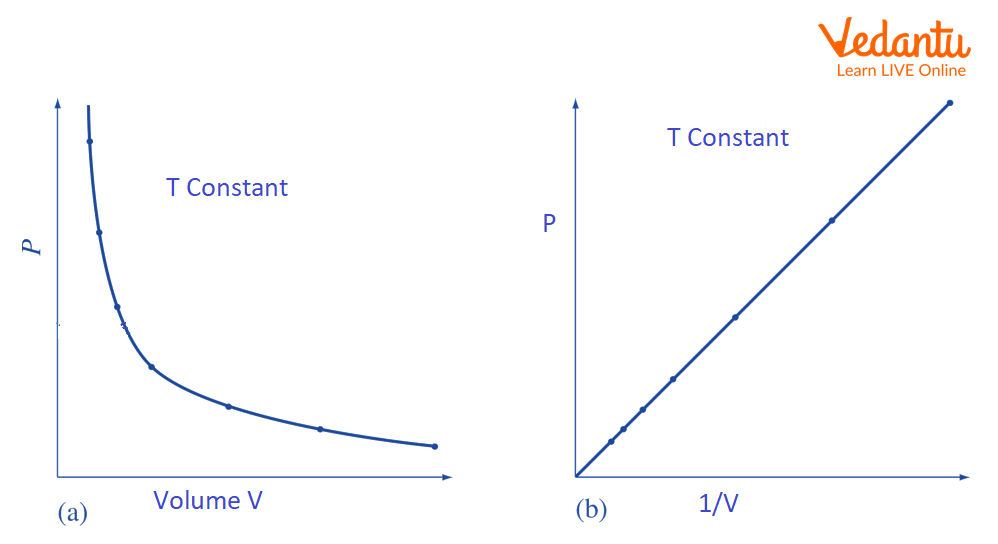
Boyle’s Graph
Precaution
When not in use, the equipment needs to be covered.
Between observations, the instrument should not be moved.
When calculating the air volume, the curved section of the closed tube needs to be corrected.
The mercury should be pure and leave no residue on the glass. When not in use, the open tube needs to be closed with cotton wool.
To measure the mercury level, the set square should be positioned tangentially to the upper meniscus.
Lab Manual Questions
1. Why would the estimation of the curved part be unreliable if tube AB has a large diameter?
Ans. It follows that the volume of the curved part will increase as tube AB's diameter increases. The error in volume will likewise increase as the volume increases. As a result, there will also be errors in length.
2. When not in use, the instrument should be covered to prevent mercury contamination of the open tube. What impact will mercury oxidation have on the experiment?
Ans. Mercury (Hg), a global pollutant, is mostly released into the atmosphere in its elemental form, Hg0, where it is oxidised to reactive HgII compounds that quickly deposit in surface ecosystems. As we are measuring the pressure in the terms of the height of the mercury column and the contamination will change the density of mercury and thus, the value of pressure.
3. Why is it necessary to precisely record using a set square the uppermost meniscus of mercury in the two tubes?
Ans. The set square is positioned tangentially to the upper meniscus of mercury when measuring it in the two tubes in order to accurately calculate its level (without error).
4. What are the assumptions made for the estimation of the volume of the curved portion of the closed tube?
Ans. In this experiment, the volume variation with pressure was examined using both methods. The first method involves increasing the volume of trapped air, and the second one involves measuring the volume of air at a specific pressure.
Viva Questions
1. How does the volume of gas change when pressure is applied to it?
Ans. When a gas is subjected to a specific amount of pressure, its volume changes correspondingly. When pressure is applied to a gas, it quickly increases, which causes the gas's volume to decrease.
2. Does Boyle's Law apply in every situation?
Ans. No, Boyle's Law is valid when the temperature and pressure are both high.
3. What kind of graph does the relationship between pressure and volume have when the temperature is constant?
Ans. The graph between pressure and volume will be hyperbolic when the temperature is constant.
4. Which gas does the experiment show Boyle's Law to be true for?
Ans. The experiment shows that Boyle's Law holds for air.
5. When the temperature is constant, how does the volume change impact the pressure?
Ans. The relation between changing volume and pressure is proportional, so a progressive reduction in the amount of gas inside can raise the pressure while an increase in volume reduces it. When examining the variation between those two factors, the process appears to be the opposite for both.
6. What is the purpose of mercury in Boyle's Law apparatus?
Ans. Mercury is used in Boyle's experiment because it expands readily when heated and prevents the gas from escaping.
7. How does the pressure change as the A and B tubes' radii change?
Ans. The radius (Area) of the tube has no effect on the pressure.
8. Why does Boyle's Law apparatus need to be vertical?
Ans. The mercury column reading won't be correct if it isn't vertical.
9. What type of graph does a gas at a constant temperature show for P vs. 1/V?
Ans. The graph of P vs. 1/V for a gas at constant temperature has the shape of a straight line.
10. What are some of the major sources of errors during the volume with pressure verification?
Ans. Significant sources of error in the verification procedure include the use of dirty air in Tube-A. The apparatus's less stable design and thin base can also be significant causes of inaccuracy.
Practical Based Questions
1. Boyle’s law is valid only for
Ideal gases
Non-ideal gases
Light Gases
Heavy Gases
Ans. (a) Boyle’s law is valid only for Ideal gases.
2. Only low-density gases obey Boyle’s and Charle’s laws. Is it true or false?
True
False
Ans. (a) Since the ideal gas law is based on the assumptions of the kinetic theory of gases, Boyle’s and Charle’s laws are used to attain the ideal gas law. Additionally, the ideal gas law only applies to gases with low density. Therefore, the above statement is true.
3. If a gas is expanded at constant temperature:
The pressure increases
The kinetic energy of the molecule decreases
The kinetic energy of the molecule remains constant
The no. of molecules of gas increases
Ans. Option b is correct as K.E =$n\,C_v\,T$ and temperature is constant,
KE= constant.
4. When the temperature remains constant, what is the name of the graph that is created?
Isotherm graph
Isobar and isochoric graph
Isochoric graph
Isobar graph
Ans. Isotherms are graphs that plot a constant temperature. For instance, the graph between pressure and volume that is used to determine Boyle's law is an isotherm because the temperature is constant in this graph.
5. What is the ideal gas equation's constant known as?
Universal gas constant
Pressure constant
Temperature constant
Boltzmann constant
Ans. PV = nRT represents the ideal gas equation, where P denotes pressure, V denotes volume, n denotes the number of moles, T is the temperature in Kelvin, and R denotes the universal gas constant.
6. If an ideal gas is placed inside a moving train after being filled in a jar, its temperature will rise.
Its temperature rises when placed inside a moving train.
Its centre of mass changes rapidly
The temperature of a moving car doesn't change.
Neither of these
Ans. If an ideal gas is placed inside a moving train after being filled in a jar, its temperature will remain constant in a moving car.
7. When gas molecules collide in a closed container, they:
Transfer momentum to the walls
Momentum becomes zero
Move-in opposite directions
Perform Brownian motion
Ans. When gas molecules collide in a closed container they transfer momentum to the walls.
8. At a specific temperature, a perfect gas's molecules have
Only potential energy
Only kinetic energy
Potential and kinetic energy both
None of the above
Ans . At a specific temperature, a perfect gas's molecules have only kinetic energy.
9. The typical force that gas exerts on a container's walls per unit area is
Temperature
Energy
Pressure
friction
Ans. The typical force that gas exerts on a container's walls per unit area is pressure.
10. The following is the law relating gas volume and pressure:
Charles' rule
The Avogadro laws
Ideal gas law
Boyle's law
Ans. The law relating gas volume and pressure is Boyle's law.
Conclusion
In this experiment, by using Boyle's apparatus a graph is plotted between pressure and volume at a constant temperature. We conclude that, under Boyle's law, the graph between P and V at constant temperature is a curve and the graph between P and V is a straight line. Also, we learnt about the relationship between the pressure and volume of a confined gas.
FAQs on Class 11 Physics To Study The Variation In Volume With Pressure For A Sample Of Air At Constant Temperature Experiment
1. What is the main objective of the experiment to study the variation of volume with pressure for air, and what crucial law does it verify for the CBSE Class 11 Physics syllabus 2025-26?
The primary objective of this experiment is to study the relationship between the pressure (P) and volume (V) of a given mass of air while keeping its temperature constant. This experiment is designed to verify Boyle's Law, which states that for a fixed mass of an ideal gas at constant temperature, the pressure is inversely proportional to the volume (P ∝ 1/V).
2. How is the graph of Pressure (P) versus the reciprocal of Volume (1/V) an important tool for verifying Boyle's Law in the practical exam?
Plotting a graph of P vs 1/V is a critical step for verification. According to Boyle's Law (PV = constant), the relationship P = k(1/V) is similar to the linear equation y = mx. Therefore, the expected graph is a straight line passing through the origin. The slope of this line represents the constant 'k' (the product of pressure and volume). A linear plot confirms the inverse relationship between P and V, thus verifying the law.
3. From an examination perspective, what are the most important precautions to take while performing the Boyle's Law experiment to ensure accurate results?
To get reliable data and marks for experimental skills, a student must observe the following precautions:
- Temperature Stability: Ensure the temperature of the enclosed air remains constant throughout the experiment. Wait for a few minutes after each compression or expansion for the air to return to room temperature.
- Airtight Apparatus: The apparatus must be perfectly airtight to ensure that the mass of the gas sample does not change.
- Parallax Error: Volume and pressure gauge readings must be taken carefully, keeping the eye level with the reading to avoid parallax error.
- Frictionless Piston: If using a piston-based apparatus, ensure it moves smoothly to apply pressure uniformly.
4. Why is the graph of P vs V for Boyle's Law a hyperbola and not a straight line?
The relationship defined by Boyle's Law is P = k/V, where 'k' is a constant. This is the equation of a rectangular hyperbola (xy = constant). A straight line represents a linear relationship (y = mx + c), which would imply that pressure changes at a constant rate with volume. However, the relationship is inverse, meaning as volume halves, pressure doubles. This non-linear, inverse relationship correctly results in a hyperbolic curve when P is plotted against V.
5. How does the behaviour of a real gas, like the air used in the experiment, deviate from the ideal behaviour predicted by Boyle's Law, especially at high pressures?
This is a higher-order thinking (HOTS) concept. Boyle's law perfectly describes an ideal gas. However, real gases like air deviate from this behaviour because Boyle's Law ignores two key factors:
- Intermolecular forces: Real gas particles attract each other, reducing the pressure compared to an ideal gas.
- Molecular volume: Real gas particles have a finite volume, which becomes significant at high pressures, making the gas less compressible than predicted.
6. Two P-V graphs (isotherms) are plotted for a gas at two different temperatures, T₁ and T₂. If T₂ > T₁, which graph will be farther from the origin and why is this an important concept?
The isotherm corresponding to the higher temperature (T₂) will be farther from the origin. This is explained by the Ideal Gas Law, PV = nRT. Since the product PV is directly proportional to the absolute temperature (T), a higher temperature results in a larger PV value. Therefore, for any given pressure or volume, the corresponding point on the T₂ curve will be further from the axes than the point on the T₁ curve.
7. What are the essential apparatus and the working formula required for the CBSE Class 11 experiment to study the variation of volume with pressure?
For this experiment, the following are typically required:
- Apparatus: A Boyle's Law apparatus, which consists of a graduated glass tube (burette or a sealed tube) connected to a reservoir of mercury or a piston system, and a pressure gauge or manometer to measure the applied pressure.
- Working Formula: The core formula to be verified is PV = constant, or P₁V₁ = P₂V₂, where P is the total pressure on the gas and V is its volume. The total pressure is often calculated as P = Atmospheric Pressure + Gauge Pressure.
























