
Write two methods of preparation of methyl alcohol. Described by diagram. Give such a test of methyl alcohol which is not given by ethyl alcohol. Give the chemical reactions.
Answer
590.1k+ views
Hint: Methyl alcohol, also known as methanol, belongs to the alcohol family and can be easily prepared from the alkyl halides (R-X), and the chemicals reactions involves the reactions with metals, Grignard reagent (RMgX), halogen acids (H-X) and phosphorus halides (P-X). Here, X represents the halogen and can be F, Cl , Br or I.
Complete step by step answer:
Alcohols are the compounds which contain the hydroxyl group(-OH) group attached to the alkyl group and are therefore regarded as the hydroxyl derivatives of hydrocarbons. They are also called aliphatic alcohols. Example: methyl alcohol, ethyl alcohol etc.
The methyl alcohol has the chemical formula as $\text{C}{{\text{H}}_{3}}\text{OH}$ and its IUPAC name is methanol and the ethyl alcohol has the chemical formula as ${{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}$ and its IUPAC name is ethanol and both of these are called as monohydric alcohols because they consists of one -OH group.
The test which is used to distinguish the methanol from ethanol is oil green test. In this test, salicylic acid is added to the methanol along with the concentrated sulphuric acid and the mixture is heated for some time and gives the odour of oil green if the only compound methanol is present due to the formation of methyl salicylate.
The reaction occurs as:
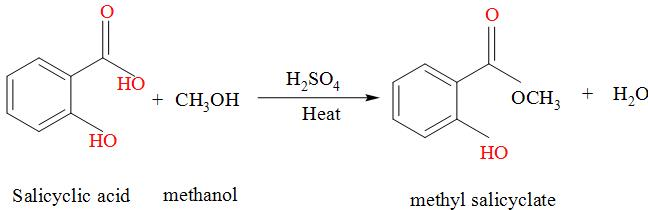
The methyl alcohol can be prepared as:
The chemicals reactions of the methyl alcohol i.e. methanol is as under:
Note: More than one OH group cannot be present on the same carbon atom and if it is present then the compound will be unstable and will readily lose water molecules to form stable aldehyde, ketone and carboxylic acid .
Complete step by step answer:
Alcohols are the compounds which contain the hydroxyl group(-OH) group attached to the alkyl group and are therefore regarded as the hydroxyl derivatives of hydrocarbons. They are also called aliphatic alcohols. Example: methyl alcohol, ethyl alcohol etc.
The methyl alcohol has the chemical formula as $\text{C}{{\text{H}}_{3}}\text{OH}$ and its IUPAC name is methanol and the ethyl alcohol has the chemical formula as ${{\text{C}}_{2}}{{\text{H}}_{5}}\text{OH}$ and its IUPAC name is ethanol and both of these are called as monohydric alcohols because they consists of one -OH group.
The test which is used to distinguish the methanol from ethanol is oil green test. In this test, salicylic acid is added to the methanol along with the concentrated sulphuric acid and the mixture is heated for some time and gives the odour of oil green if the only compound methanol is present due to the formation of methyl salicylate.
The reaction occurs as:

The methyl alcohol can be prepared as:
| Sr.no | Method | Formation of methyl alcohol |
| 1. | From haloalkanes: in this method, haloalkanes i.e. X-R reacts with the moist silver oxide (AgOH) to give methyl alcohol. | The reaction occurs as: \[\begin{align} & \text{C}{{\text{H}}_{3}}\text{I + AgOH}\to \text{ C}{{\text{H}}_{3}}\text{OH +AgI} \\ & \text{Iodomethane moist methanol} \\ & \text{ silver oxide} \\ \end{align}\] |
| 2. | From dimethyl ether: dimethyl ether on reaction with the hot sulphuric acid undergoes hydrolysis and gives methanol. | The reaction occurs as: \[\] \[\begin{align} & \text{C}{{\text{H}}_{{}}}\text{-O-C}{{\text{H}}_{3}}\text{ +}{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}\text{ }\to \text{ 2C}{{\text{H}}_{3}}\text{OH} \\ & \text{dimethyl dil sulphuric methanol} \\ & \text{ ether acid } \\ \end{align}\] |
The chemicals reactions of the methyl alcohol i.e. methanol is as under:
| 1. | Reaction with metals:- methanol reacts with metals like sodium , potassium etc. to form metal oxides and the hydrogen gas. | The reaction occurs as: \[\begin{align} & \text{2C}{{\text{H}}_{3}}\text{OH + 2Na}\to \text{2CH}{{\text{O}}^{-}}\text{N}{{\text{a}}^{+}}\text{ +}{{\text{H}}_{2}} \\ & \text{Methanol sodium } \\ & \text{ methoxide} \\ \end{align}\] |
| 2. | Reaction with Grignard reagent:- methanol reacts with the Grignard reagent to form alkanes. | The reaction occurs as: \[\begin{align} & \text{C}{{\text{H}}_{3}}\text{OH + C}{{\text{H}}_{3}}\text{C}{{\text{H}}_{3}}\text{MgBr }\to \text{C}{{\text{H}}_{3}}\text{C}{{\text{H}}_{3}}\text{ +C}{{\text{H}}_{3}}\text{OMgBr} \\ & \text{Methanol Ethyl magnesium ethane methyl mag}\text{.} \\ & \text{ bromide bromide} \\ \end{align}\] |
| 3. | Reaction with halogen acids:- methanol reacts with halogen acids i.e. HI to form alkyl iodides. | The reaction occurs as: \[\begin{align} & \text{C}{{\text{H}}_{3}}\text{OH + HI }\to \text{ C}{{\text{H}}_{3}}\text{I + }{{\text{H}}_{2}}\text{O} \\ & \text{methanol hydrogen Iodomethane} \\ & \text{ iodide} \\ \end{align}\] |
| 4. | Reaction with phosphorus halides:- methanol reacts with phosphorus halides i.e.$\text{P}{{\text{I}}_{3}}\text{,PC}{{\text{l}}_{5}}\text{,PC}{{\text{l}}_{3}}$etc. to form alkyl halides. | The reaction occurs as: \[\begin{align} & \text{3C}{{\text{H}}_{3}}\text{OH + P}{{\text{I}}_{3}}\text{ }\to \text{ 3C}{{\text{H}}_{3}}\text{I + }{{\text{H}}_{3}}\text{P}{{\text{O}}_{3}} \\ & \text{Methanol Iodomethane phosphorous} \\ & \text{ acid} \\ \end{align}\] |
Note: More than one OH group cannot be present on the same carbon atom and if it is present then the compound will be unstable and will readily lose water molecules to form stable aldehyde, ketone and carboxylic acid .
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




