
Write the types of isomerism exhibited by the following complexes.
A) $\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]S{O_4}$
B) ${\left[ {Co{{\left( {e{n_{}}} \right)}_3}} \right]^{3 + }}$
C) $\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Cr{{\left( {CN} \right)}_6}} \right]$
Answer
560.7k+ views
Hint: Ionization isomerism- Isomerism exhibited by compounds which give different ions in solution although they have the same composition.
Optical isomerism- This isomerism is exhibited by compounds which are non-superimposable mirror images of each other.
Coordination isomerism- This type of isomerism is possible when both positive and negative ions of a salt are complex ions and the two isomers differ in the distribution of ligands in cation and anion.
Complete answer:
$\left( i \right)$ Ionization isomerism is exhibited by compound $\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]S{O_4}$
The isomers obtained are $\left[ {Co{{\left( {N{H_3}} \right)}_5}C{l^{}}} \right]S{O_4}and\,\left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]Cl$
As you can clearly see they only differ in position of ions in the inner and outer coordination sphere.
$\left( {ii} \right)$Optical isomerism is exhibited by the compound ${\left[ {Co{{\left( {e{n_{}}} \right)}_3}} \right]^{3 + }}$
As you can see that these two structures have an enantiomeric relationship between them i.e., non-superimposable mirror image relationship.
.
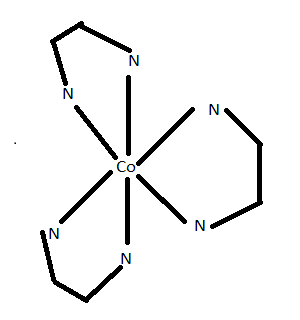
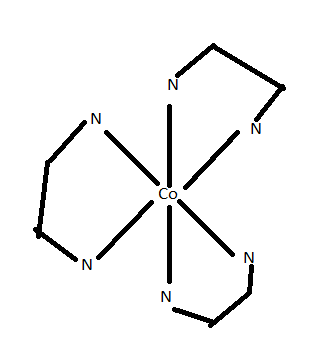
.
Dextro and laevo are two forms for this compound.
$\left( {iii} \right)$ Coordination isomerism is exhibited by the compound $\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Cr{{\left( {CN} \right)}_6}} \right]$
$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Co{{\left( {CN} \right)}_6}} \right]$and $\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Cr{{\left( {CN} \right)}_6}} \right]$ are both coordination isomers of the compound where we can see the ligands are exchanged between the two coordination spheres.
Note:Optical isomerism is shown by complexes whose mirror images are non-super imposable on each other.Ionization isomerism is shown by complexes which give different ions in solution on hydrolysis.Coordination isomerism is shown by complexes having both cationic and anionic part as complex ions and differ in distribution of ligands in cationic and anionic complex parts.
Optical isomerism- This isomerism is exhibited by compounds which are non-superimposable mirror images of each other.
Coordination isomerism- This type of isomerism is possible when both positive and negative ions of a salt are complex ions and the two isomers differ in the distribution of ligands in cation and anion.
Complete answer:
$\left( i \right)$ Ionization isomerism is exhibited by compound $\left[ {Co{{\left( {N{H_3}} \right)}_5}Cl} \right]S{O_4}$
The isomers obtained are $\left[ {Co{{\left( {N{H_3}} \right)}_5}C{l^{}}} \right]S{O_4}and\,\left[ {Co{{\left( {N{H_3}} \right)}_5}S{O_4}} \right]Cl$
As you can clearly see they only differ in position of ions in the inner and outer coordination sphere.
$\left( {ii} \right)$Optical isomerism is exhibited by the compound ${\left[ {Co{{\left( {e{n_{}}} \right)}_3}} \right]^{3 + }}$
As you can see that these two structures have an enantiomeric relationship between them i.e., non-superimposable mirror image relationship.
.


.
Dextro and laevo are two forms for this compound.
$\left( {iii} \right)$ Coordination isomerism is exhibited by the compound $\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Cr{{\left( {CN} \right)}_6}} \right]$
$\left[ {Cr{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Co{{\left( {CN} \right)}_6}} \right]$and $\left[ {Co{{\left( {N{H_3}} \right)}_6}} \right]\left[ {Cr{{\left( {CN} \right)}_6}} \right]$ are both coordination isomers of the compound where we can see the ligands are exchanged between the two coordination spheres.
Note:Optical isomerism is shown by complexes whose mirror images are non-super imposable on each other.Ionization isomerism is shown by complexes which give different ions in solution on hydrolysis.Coordination isomerism is shown by complexes having both cationic and anionic part as complex ions and differ in distribution of ligands in cationic and anionic complex parts.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




