
Write the structures of the following organic compounds
i.1-bromo-4-sec-butyl-2-methylbenzene
ii.2-chloro-1-phenyl butane
iii.p-bromochlorobenzene
iv.4-t-butyl-3-iodoheptane
Answer
502.5k+ views
Hint: We have to know that in order to find out the structure of the given compounds, one must be familiar with the naming scheme of IUPAC (International Union of Pure and Applied Chemistry). This nomenclature with specific rules provides unique, self-explanatory names for chemical compounds.
Complete answer:
Following are the procedure to draw the given IUPAC structures:
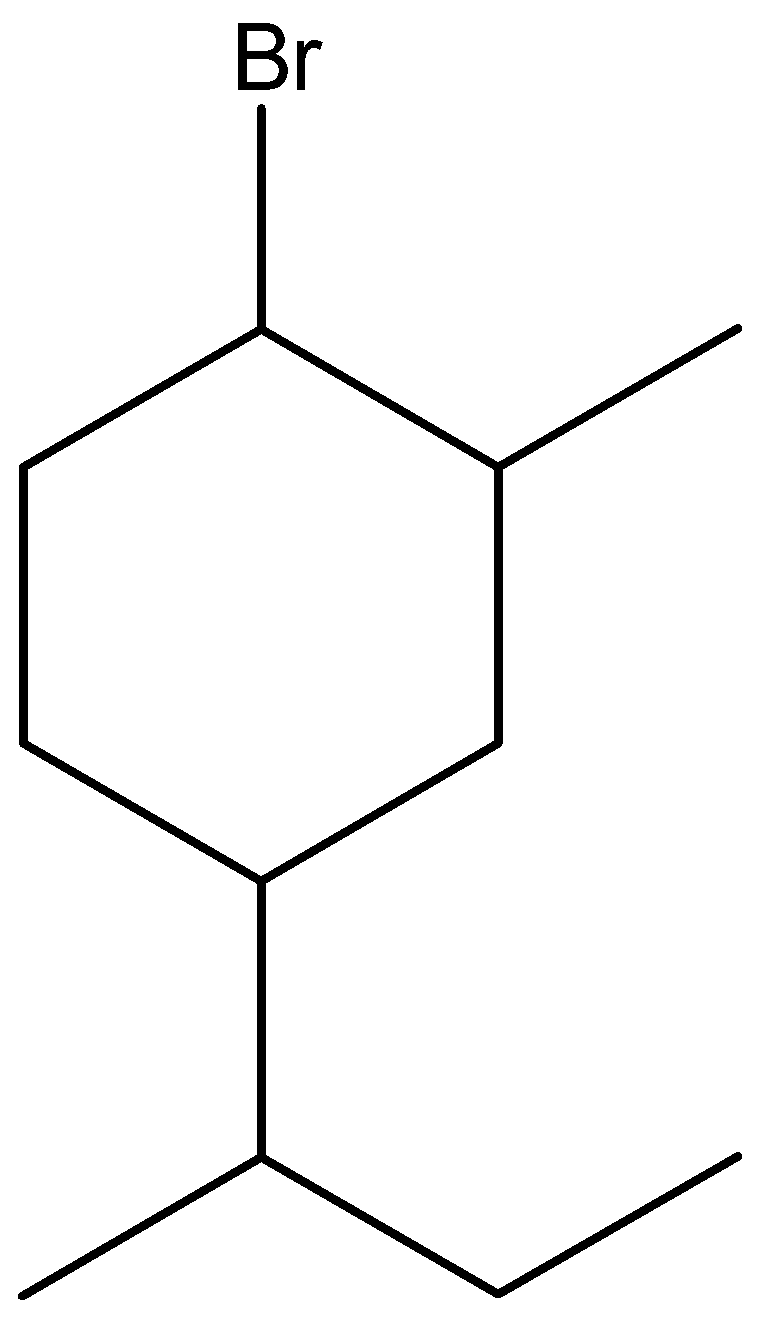
i.1-bromo-4-sec-butyl-2-methylbenzene
The parent chain of this compound is benzene. Draw a benzene ring and replace hydrogen atom from C1 of benzene with bromine, C2 with a methyl group. Then add secondary butane (also known as 1-methyl propyl) to C4 of the benzene.
ii.2-chloro-1-phenyl butane
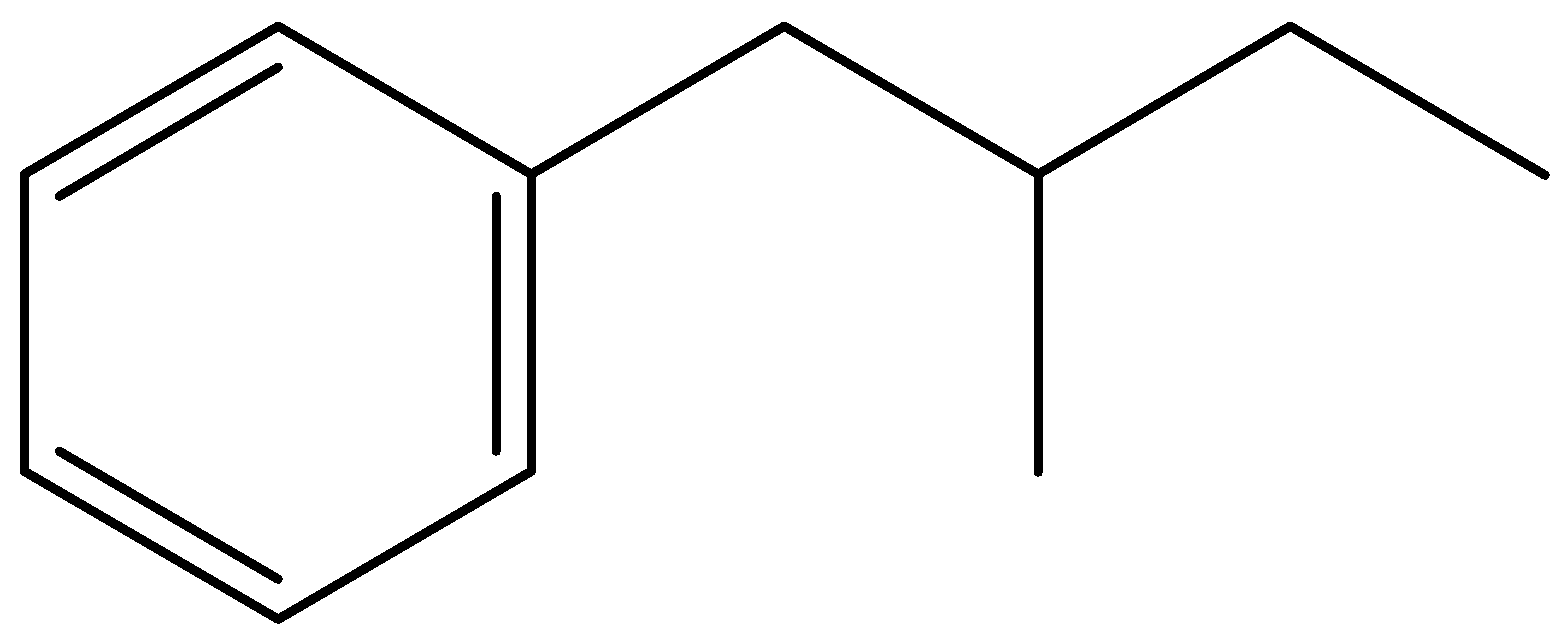
In this compound, the phenyl ring is the parent chain. Draw a benzene ring, and replace one hydrogen atom with a functional group to obtain a phenyl group. Here the functional group is butane. The carbon atom of butane directly attached to the phenyl ring is numbered as C1. Then remove one hydrogen from C2 of butane and add chlorine to form 2-Chloro-1-phenyl butane.
 .
.
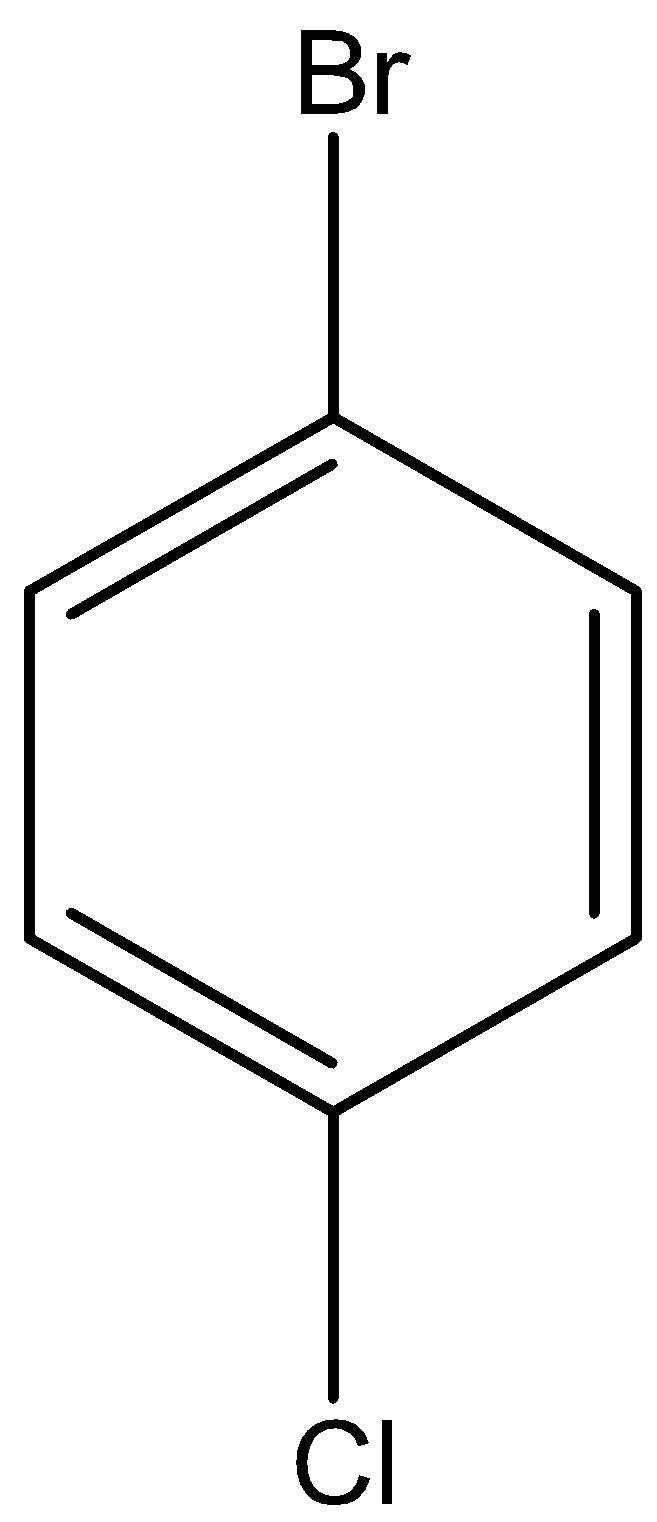
iii.p-bromochlorobenzene
This is a simple structure with benzene as the parent chain. It is also known as 1-bromo-4 chlorobenzene. So, replace hydrogen atoms from C1 of benzene with bromine and C4 with chlorine atoms to obtain p-bromochlorobenzene.
iv.4-t-butyl-3-iodoheptane
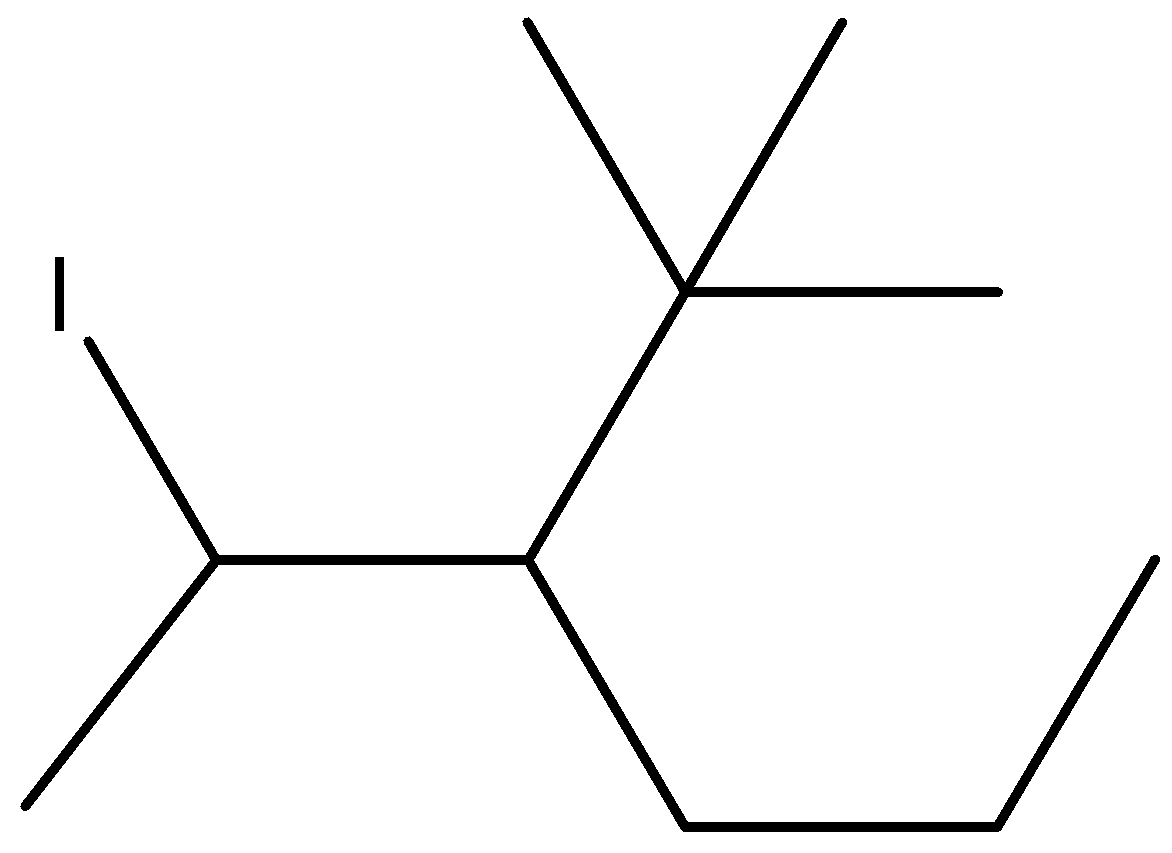
Here, the parent chain is a seven carbon long heptane chain. The chain will be numbered in such a way that the branched-chain and functional group get the lowest number. Replace hydrogen atoms from C2 with iodine and C3 with tertiary butane, which is also known as 1,1-dimethyl ethyl.
Note:
We must know that to draw an IUPAC structure, identify and draw the parent chain with the highest number of carbon atoms and number the carbon atoms unidirectionally. Then add functional groups mentioned at the specific positions.
Complete answer:
Following are the procedure to draw the given IUPAC structures:
i.1-bromo-4-sec-butyl-2-methylbenzene
The parent chain of this compound is benzene. Draw a benzene ring and replace hydrogen atom from C1 of benzene with bromine, C2 with a methyl group. Then add secondary butane (also known as 1-methyl propyl) to C4 of the benzene.

ii.2-chloro-1-phenyl butane
In this compound, the phenyl ring is the parent chain. Draw a benzene ring, and replace one hydrogen atom with a functional group to obtain a phenyl group. Here the functional group is butane. The carbon atom of butane directly attached to the phenyl ring is numbered as C1. Then remove one hydrogen from C2 of butane and add chlorine to form 2-Chloro-1-phenyl butane.

iii.p-bromochlorobenzene
This is a simple structure with benzene as the parent chain. It is also known as 1-bromo-4 chlorobenzene. So, replace hydrogen atoms from C1 of benzene with bromine and C4 with chlorine atoms to obtain p-bromochlorobenzene.

iv.4-t-butyl-3-iodoheptane
Here, the parent chain is a seven carbon long heptane chain. The chain will be numbered in such a way that the branched-chain and functional group get the lowest number. Replace hydrogen atoms from C2 with iodine and C3 with tertiary butane, which is also known as 1,1-dimethyl ethyl.

Note:
We must know that to draw an IUPAC structure, identify and draw the parent chain with the highest number of carbon atoms and number the carbon atoms unidirectionally. Then add functional groups mentioned at the specific positions.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




