
Write the IUPAC name of the following:

Answer
582.6k+ views
Hint: The IUPAC naming which associates the aldehyde group in the molecule have some special notified methods. The identification is the most vital step while naming the compound.
Complete step by step solution:
Let us understand the IUPAC culture of naming the compounds on some terms and conditions.
IUPAC-
A systematic method of naming organic compounds is recommended by the International Union of Pure and Applied Chemistry which is known as IUPAC nomenclature.
The steps for naming any compound is as follows;
1. Identification of basic parent chain to which the substitutes are attached.
2. Parent functional group identification.
3. Other functional groups are identified.
4. See whether the compound has a single, double or triple bond in it.
5. Side chains attached to the parent chain are recognised and numbering is done.
Our given data focuses on the aldehyde group, so let’s see about the aldehyde functional group.
Aldehydes are named by replacing the suffix -ane by -anal.
Presence of more than one aldehyde group results in addition in suffix i.e. -anedial for 2 groups.
That’s not necessary to put the number to indicate the aldehyde group as it would always be at the end of the chain and would be assigned as C-1.
Thus, for given two compounds the IUPAC names can be given with the help of above-mentioned rules;
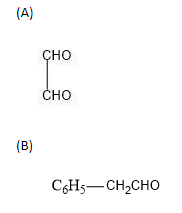
IUPAC name of
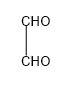
is Ethanedial as it consists of 2 carbon atoms which are aldehyde groups in themselves.
Whereas, IUPAC name of
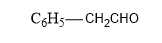
is 2-phenylacetaldehyde as the parent chain consists of the phenyl group and at the second position aldehyde group is attached.
Note: Note that there are many names given to a single compound during its nomenclature and priorities. But we should always study and use the preferred IUPAC name of any of the compounds.
Complete step by step solution:
Let us understand the IUPAC culture of naming the compounds on some terms and conditions.
IUPAC-
A systematic method of naming organic compounds is recommended by the International Union of Pure and Applied Chemistry which is known as IUPAC nomenclature.
The steps for naming any compound is as follows;
1. Identification of basic parent chain to which the substitutes are attached.
2. Parent functional group identification.
3. Other functional groups are identified.
4. See whether the compound has a single, double or triple bond in it.
5. Side chains attached to the parent chain are recognised and numbering is done.
Our given data focuses on the aldehyde group, so let’s see about the aldehyde functional group.
Aldehydes are named by replacing the suffix -ane by -anal.
Presence of more than one aldehyde group results in addition in suffix i.e. -anedial for 2 groups.
That’s not necessary to put the number to indicate the aldehyde group as it would always be at the end of the chain and would be assigned as C-1.
Thus, for given two compounds the IUPAC names can be given with the help of above-mentioned rules;
IUPAC name of

is Ethanedial as it consists of 2 carbon atoms which are aldehyde groups in themselves.
Whereas, IUPAC name of
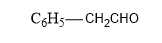
is 2-phenylacetaldehyde as the parent chain consists of the phenyl group and at the second position aldehyde group is attached.
Note: Note that there are many names given to a single compound during its nomenclature and priorities. But we should always study and use the preferred IUPAC name of any of the compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




