
Write the IUPAC name of the complex\[{\left[ {Cr{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]^ + }\]. What type of isomerism does it exhibit?
Answer
573.9k+ views
Hint: IUPAC stands for International Union of Pure and Applied Chemistry. It is the world authority on the chemical nomenclature as well as terminology. Thus, IUPAC nomenclature is basically a method of naming coordination or organic compounds according to certain rules.
Complete answer
IUPAC provides consistency to the names of coordination or organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names.
A complex is basically a substance in which a metal ion or atom is linked with either a group of neutral molecules or anions known as ligands. And coordination compounds are the neutral substances (means no charge) in which at least one ion is present in the form of a complex. The coordination compounds can be named in the following manner:
In order to assign a name to a coordination compound, always name cation before anion. It doesn’t matter whether the complex ion is cation or anion.
In order to assign a name to the complex ion, assign name to the ligands first in an alphabetical order, then metal ion or atom. It should be kept in mind that the metal ion or atom is always written before ligands in the chemical formula.
In the given compound, there are four neutral ligands i.e. ammine and 2 anionic ligands i.e. chloride are present and central metal cation is chromium. Thus, IUPAC name becomes Tetraamminedichlorochromium(III) ion.
Hence, the IUPAC name of the given complex \[{\left[ {Cr{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]^ + }\] is Tetraamminedichlorochromium(III) ion.
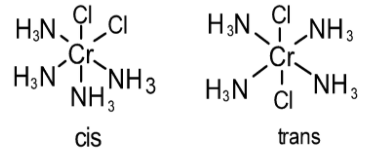
It exhibits geometrical isomerism (i.e. cis trans isomerism). cis isomer possess two chloride ligands which are adjacent to each other while trans isomer possess two chloride ligands that are opposite to each other as shown below:
Note:
Two main types of isomerism include structural or constitutional isomerism which differ in terms of the bonds between the atoms and the other type of isomerism includes stereoisomerism or spatial isomerism which differ in terms of the relative positions of the atoms but the bonds are same.
Complete answer
IUPAC provides consistency to the names of coordination or organic compounds. It enables every compound to possess a unique name, which otherwise is not plausible with the common names.
A complex is basically a substance in which a metal ion or atom is linked with either a group of neutral molecules or anions known as ligands. And coordination compounds are the neutral substances (means no charge) in which at least one ion is present in the form of a complex. The coordination compounds can be named in the following manner:
In order to assign a name to a coordination compound, always name cation before anion. It doesn’t matter whether the complex ion is cation or anion.
In order to assign a name to the complex ion, assign name to the ligands first in an alphabetical order, then metal ion or atom. It should be kept in mind that the metal ion or atom is always written before ligands in the chemical formula.
In the given compound, there are four neutral ligands i.e. ammine and 2 anionic ligands i.e. chloride are present and central metal cation is chromium. Thus, IUPAC name becomes Tetraamminedichlorochromium(III) ion.
Hence, the IUPAC name of the given complex \[{\left[ {Cr{{\left( {N{H_3}} \right)}_4}C{l_2}} \right]^ + }\] is Tetraamminedichlorochromium(III) ion.
It exhibits geometrical isomerism (i.e. cis trans isomerism). cis isomer possess two chloride ligands which are adjacent to each other while trans isomer possess two chloride ligands that are opposite to each other as shown below:

Note:
Two main types of isomerism include structural or constitutional isomerism which differ in terms of the bonds between the atoms and the other type of isomerism includes stereoisomerism or spatial isomerism which differ in terms of the relative positions of the atoms but the bonds are same.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




