
Write the IUPAC name of $C{H_3} - C{H_2} - C{H_2} - CH = C{H_2}$.
Answer
581.1k+ views
Hint: When two pairs of electrons are shared by the atom to form a chemical bond, the resulting bond will be a double bond. The hydrocarbon comprising a double bond between the two groups is termed as alkenes.
Complete step by step answer:
The full form of IUPAC is the International Union of pure and applied chemistry. The system has set some rules for naming the chemical compounds.
The given compound is shown below.
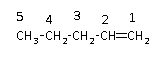
STEP 1: Identify the group
In the given hydrocarbon, a double bond is present in the first position of the parent chain due to which the suffix will be –ene.
STEP 2: Find the longest carbon chain
The hydrocarbon consists of five carbon atoms in the longest chain or parent chain. Therefore, the hydrocarbon is named as pentane.
STEP 3: Number the carbon atoms present in the longest chain
The numbering of the carbon atom starts from the double bond as it is the highest priority group.
STEP 4: Combine the entire element into a single IUPAC name
The IUPAC name of the compound is pent-1-ene.
Additional information: The aliphatic hydrocarbon is divided into straight-chain and branched-chain. Both of them have the same general formula ${C_n}{H_{2n + 2}}$. In a straight chain, the carbon atom is bonded with two hydrogen atoms from both the side and in branched-chain, removal of one hydrogen from the $ - C{H_2}$ group takes place which is replaced by an alkyl group.
Note:
In the IUPAC name, the number represents the position of the group or any other functional group and it is written before the suffix. Alkenes are also termed as olefins.
Complete step by step answer:
The full form of IUPAC is the International Union of pure and applied chemistry. The system has set some rules for naming the chemical compounds.
The given compound is shown below.
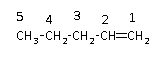
STEP 1: Identify the group
In the given hydrocarbon, a double bond is present in the first position of the parent chain due to which the suffix will be –ene.
STEP 2: Find the longest carbon chain
The hydrocarbon consists of five carbon atoms in the longest chain or parent chain. Therefore, the hydrocarbon is named as pentane.
STEP 3: Number the carbon atoms present in the longest chain
The numbering of the carbon atom starts from the double bond as it is the highest priority group.
STEP 4: Combine the entire element into a single IUPAC name
The IUPAC name of the compound is pent-1-ene.
Additional information: The aliphatic hydrocarbon is divided into straight-chain and branched-chain. Both of them have the same general formula ${C_n}{H_{2n + 2}}$. In a straight chain, the carbon atom is bonded with two hydrogen atoms from both the side and in branched-chain, removal of one hydrogen from the $ - C{H_2}$ group takes place which is replaced by an alkyl group.
Note:
In the IUPAC name, the number represents the position of the group or any other functional group and it is written before the suffix. Alkenes are also termed as olefins.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




