
Write IUPAC names of the products obtained by the ozonolysis of the pent-2-ene?
Answer
567.3k+ views
Hint: Understand the reaction of ozonolysis. Ozonolysis is primarily used to form carbonyl compounds. Based on this try to devise a possible mechanism for ozonolysis. Now identify the products formed and name them in accordance with IUPAC nomenclature for organic compounds.
Complete Solution :
Ozonolysis is an organic reaction where the unsaturated bonds of alkenes or other unsaturated compounds are cleaved using ozone as the reagent.
- Alkenes as well as alkynes form compounds such that the multiple bond system is replaced by carbonyl group thus forming either ketones or aldehydes. On the other hand, a multiple bond system containing nitrogen, forms nitrosamines.
- We will now write the ozonolysis reaction of pent-2-ene.
Ozone being thermodynamically unstable, attacks the double bond forming an intermediate called ozonide structure.
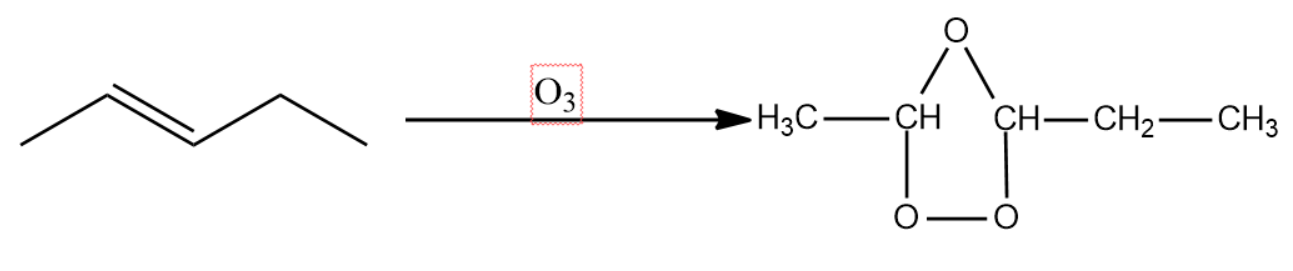
This intermediate is then exposed to zinc metal in the presence of metal. This leads to the formation of two carbonyl compounds by the homolytic fission of the C-O-C bond.
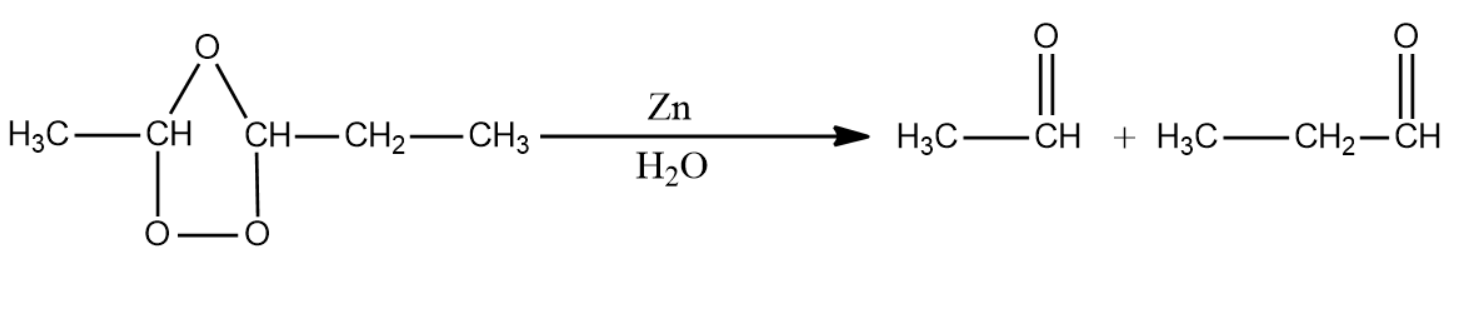
The IUPAC name for the products formed is ethanal and propanal.
So, the correct answer is “Option A”.
Additional information: The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in the individual countries. IUPAC is registered in Zürich; Switzerland and its administrative office is called IUPAC secretariat.
Note: Ozonolysis can be oxidative in nature as well. By this it means that instead of Zn and water, if we had used hydrogen peroxide, we would have got their respective acids i.e. ethanoic acid and propanoic acid. Reductive ozonolysis is carried out in the reaction above keeping in mind the options given.
Complete Solution :
Ozonolysis is an organic reaction where the unsaturated bonds of alkenes or other unsaturated compounds are cleaved using ozone as the reagent.
- Alkenes as well as alkynes form compounds such that the multiple bond system is replaced by carbonyl group thus forming either ketones or aldehydes. On the other hand, a multiple bond system containing nitrogen, forms nitrosamines.
- We will now write the ozonolysis reaction of pent-2-ene.
Ozone being thermodynamically unstable, attacks the double bond forming an intermediate called ozonide structure.

This intermediate is then exposed to zinc metal in the presence of metal. This leads to the formation of two carbonyl compounds by the homolytic fission of the C-O-C bond.

The IUPAC name for the products formed is ethanal and propanal.
So, the correct answer is “Option A”.
Additional information: The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations that represents chemists in the individual countries. IUPAC is registered in Zürich; Switzerland and its administrative office is called IUPAC secretariat.
Note: Ozonolysis can be oxidative in nature as well. By this it means that instead of Zn and water, if we had used hydrogen peroxide, we would have got their respective acids i.e. ethanoic acid and propanoic acid. Reductive ozonolysis is carried out in the reaction above keeping in mind the options given.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




