
Write about hydrogen bonding in ice and water.
Answer
572.1k+ views
Hint: Water is in liquid state and ice is its solid state. In solids the packing of molecules is tight as compared to liquid and gas. Hydrogen bonding occurs between hydrogen and strongly electronegative atoms like F, N, O. In water hydrogen bonding occurs as there is hydrogen as well as oxygen both are present.
Complete Step by step answer: the formation of hydrogen bonds is an important quality of liquid water. As hydrogen bonding occurs between water molecules, therefore it has some unique properties than other liquids. And because of these unique chemical and physical properties of water, it is known as a universal liquid and has a crucial role in the biological system.
Water has 2 hydrogen atoms and 1 oxygen atom. The oxygen of one water molecule has two lone pairs of electrons, each of which can form hydrogen bonds with hydrogen on other two water molecules.
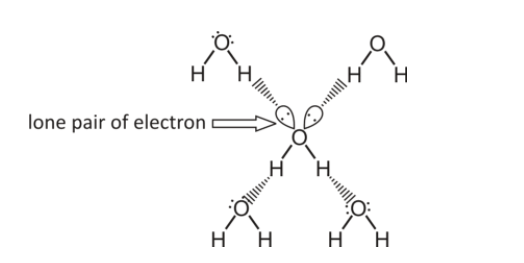
The above figure shows Hydrogen bonding in water (here the solid line denotes the covalent bond and dashes line denote hydrogen bonds)
As water is boiled, kinetic energy causes the hydrogen bonds to break and allow water molecules to escape into air and form steam.
When water freezes, water molecules form a crystalline structure which is formed with the help of hydrogen bonding.
In ice each water molecule is hydrogen bonded to four neighboring water molecules and forms a crystalline structure. In ice these hydrogen bonds are stable. This type of crystalline array of hydrogen bond leaves a space between the water molecules and these water molecules are now farther apart than they were in liquid water. This leads to a decrease in the density of water in its frozen form. Or we can say hydrogen bonds enable the ice to float in water, because of the spacing caused by hydrogen bonds.
Note: Hydrogen bonding can be intermolecular or intramolecular. If the hydrogen bonding occurs between the molecules then it is intermolecular like in water, but if the hydrogen bond forms within the molecule then it is intramolecular in nature.
Complete Step by step answer: the formation of hydrogen bonds is an important quality of liquid water. As hydrogen bonding occurs between water molecules, therefore it has some unique properties than other liquids. And because of these unique chemical and physical properties of water, it is known as a universal liquid and has a crucial role in the biological system.
Water has 2 hydrogen atoms and 1 oxygen atom. The oxygen of one water molecule has two lone pairs of electrons, each of which can form hydrogen bonds with hydrogen on other two water molecules.

The above figure shows Hydrogen bonding in water (here the solid line denotes the covalent bond and dashes line denote hydrogen bonds)
As water is boiled, kinetic energy causes the hydrogen bonds to break and allow water molecules to escape into air and form steam.
When water freezes, water molecules form a crystalline structure which is formed with the help of hydrogen bonding.
In ice each water molecule is hydrogen bonded to four neighboring water molecules and forms a crystalline structure. In ice these hydrogen bonds are stable. This type of crystalline array of hydrogen bond leaves a space between the water molecules and these water molecules are now farther apart than they were in liquid water. This leads to a decrease in the density of water in its frozen form. Or we can say hydrogen bonds enable the ice to float in water, because of the spacing caused by hydrogen bonds.
Note: Hydrogen bonding can be intermolecular or intramolecular. If the hydrogen bonding occurs between the molecules then it is intermolecular like in water, but if the hydrogen bond forms within the molecule then it is intramolecular in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




